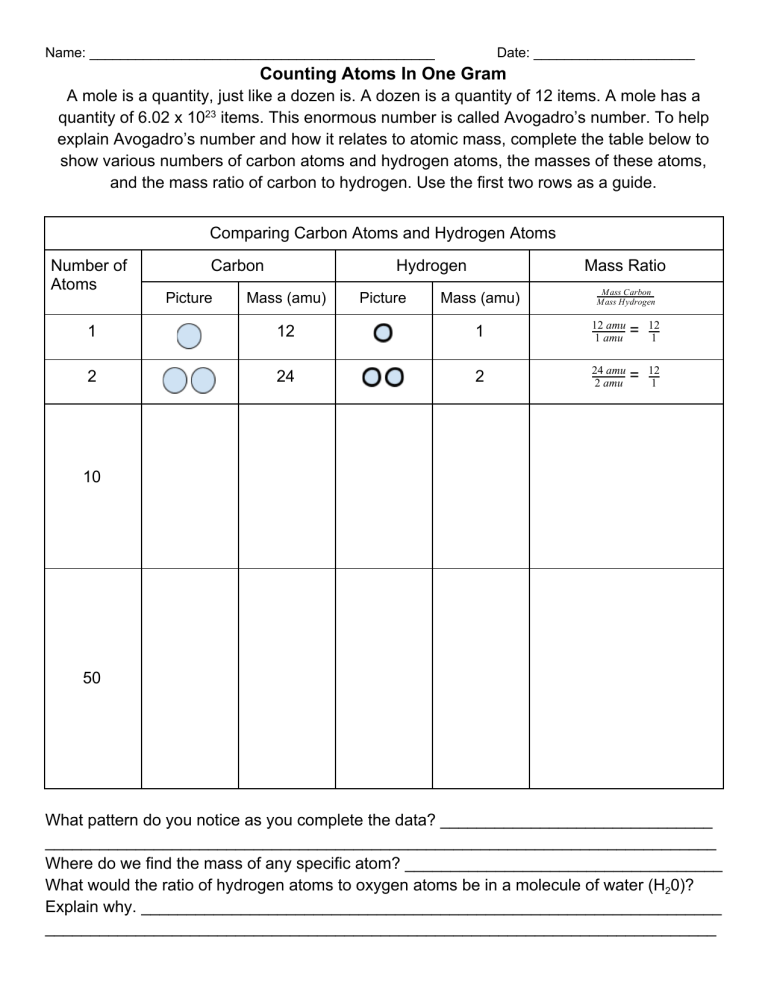
Name: _____________________________________________ Date: _____________________ Counting Atoms In One Gram A mole is a quantity, just like a dozen is. A dozen is a quantity of 12 items. A mole has a quantity of 6.02 x 1023 items. This enormous number is called Avogadro’s number. To help explain Avogadro’s number and how it relates to atomic mass, complete the table below to show various numbers of carbon atoms and hydrogen atoms, the masses of these atoms, and the mass ratio of carbon to hydrogen. Use the first two rows as a guide. Comparing Carbon Atoms and Hydrogen Atoms Number of Atoms Carbon Picture Hydrogen Mass (amu) Picture Mass Ratio Mass (amu) M ass Carbon M ass Hydrogen 1 12 1 12 amu 1 amu = 12 1 2 24 2 24 amu 2 amu = 12 1 10 50 What pattern do you notice as you complete the data? ______________________________ __________________________________________________________________________ Where do we find the mass of any specific atom? ___________________________________ What would the ratio of hydrogen atoms to oxygen atoms be in a molecule of water (H20)? Explain why. ________________________________________________________________ __________________________________________________________________________ Name: _____________________________________________ Date: _____________________ When reporting molecular masses, you should give the answer with the units in amu, but if giving the molar mass, you will need to provide an answer with units of g/mol. Ex: The molecular mass of sodium is 23 amu, but its molar mass is 23 g/mol. Practice identifying molar mass for elements and for compounds by completing the practice problems below: What is the molar mass for each element below? 1. Lithium - ________________ 2. Copper - ________________ 3. Gold - __________________ 4. Aluminum - ______________ What is the molar mass of each compound below? Show your work, and round to the appropriate amount of significant figures. 1. NaBr - Mass of Na: __________ Mass of Br: _________ Molar Mass: _________g/mol 2. PbSO4 3. Ca(OH)2 - 4. Na3PO4 5. (NH4)2CO3 6. C6H12O6 7. Fe3(PO4)2 8. (NH4)2S 9. Zn(C2H3O2)2 10. AgF - 11. AlF3 -





