File - Mrs. Dawson's Classroom
advertisement
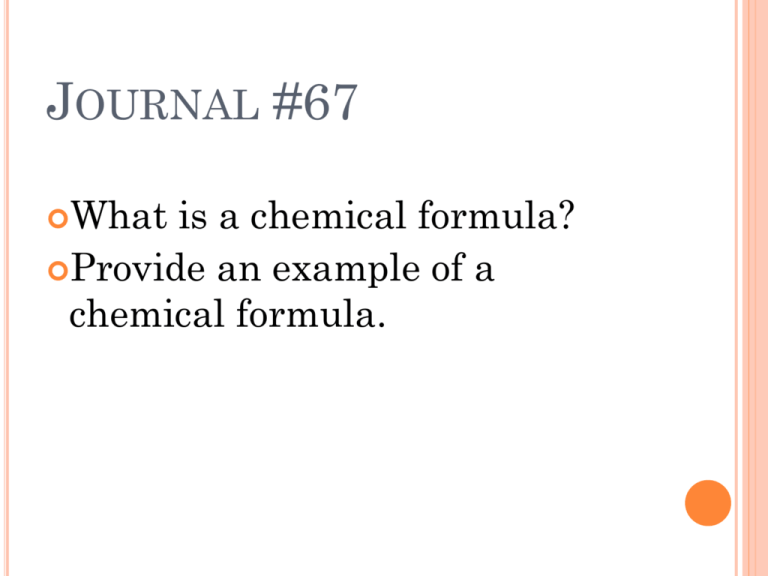
JOURNAL #67 What is a chemical formula? Provide an example of a chemical formula. TODAY WE WILL LEARN HOW TO USE CHEMICAL FORMULAS MOLAR MASS REVIEW: Give the molar mass of the following elements: Na 2. C 3. O 4. Xe 1. CAN YOU GUESS THE COMPOUND? 1. 2. H2O NaCl MOLAR MASS OF A FORMULA MASSES Previously, we saw that hydrogen atoms have an average atomic mass of 1.00794 amu and oxygen has an average atomic mass of 15.9994 amu. Like individual atoms, molecules or ions have characteristic average masses Example: H2O is composed of 2 hydrogens and 1 oxygen. The average atomic mass can be calculated: 2 H atoms x 1.01 amu = 2.02 amu H atom 1 O atom x 16.00 amu= 16.00 amu O atom average atomic mass of H20 = 18.02 amu FORMULA MASS The mass of a water molecule can be referred to as a molecular mass. The mass of one NaCl formula unit, is not a molecular mass because NaCl is an ionic compound. The mass of any unit represented by a chemical formula, where the unit is a molecule, a formula unit, or an ion is known as the formula mass. FIND THE FORMULA MASS OF THE FOLLOWING: 1. 2. 3. 4. 5. KClO3 H2SO4 Ca(NO3)2 PO43MgCl2 MOLAR MASS Molar mass of a substance is qual to the mass in grams of one mole (or 6.022 x 10 ^23 particles) Example: molar mass of Ca is 40.08g/mol because calcium atoms has a mass of 40.08g The molar mass of a compound is calculated by summing the masses of the elements present in a mole of the molecules or formula units that make up the compound. MOLAR MASS A compounds molar mass is numerically equal to its formula mass. Example: 2 H atoms x 1.01 g H 1 mol H 1 O atom x 16.00 g O 1 mol O molar mass of H20 = 2.02 g H = 16.00 g O = 18.02 g/mol WHAT IS THE MOLAR MASS OF THE FOLLOWING: 1. 2. 3. 4. Ba(NO3)2 Al2S3 NaNO3 Ba(OH)2 CONVERSION FACTOR Molar mass of a compound can be used as a conversion factor to relate the amount in moles to a mass in grams for a given substance. To convert a known amount of a compound in moles to a mass in grams, multiply the amount in moles by the molar mass. Example: what is the mass in grams of 2.50 mol of oxygen gas? EXAMPLE: Ibuprofen, C13H18O2 is the active ingredient in many nonprescription pain relievers. Its molar mass is 206.31g/mol. If the tablets in a bottle contain a total of 33g of ibuprofen, how many moles of ibuprofen are in the bottle? How many molecules of ibuprofen are in the bottle? What is the total mass in grams of carbon in 33g of ibuprofen?



