Biology Test 2 Review - Petoskey Public Schools
advertisement

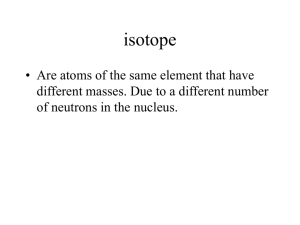
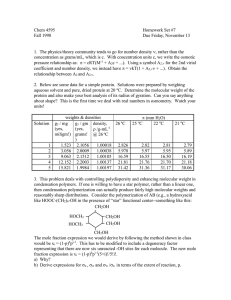
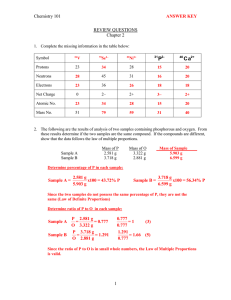
Review: Chemistry TEST 2: Primarily Chapter 3 Problems & Questions: 1 Who are the major historical figures central to discovering the existence and structure of the atom and their major contributions? 2 Draw a chart showing the charge, mass, location and symbol of protons, neutrons, and electrons 3a Atomic Number = 3b Mass Number= 4 Electron energy levels fill from to 5 How many electrons can be on each electron energy level (Levels 1-6)? 6 What cloud shape does s, p, and d orbital have and how many electrons fit into each? 7 Explain what the strong force is and the purpose of it. 8 What is an isotope? 9 What is an ion? 10 How are ions formed? 11 How can you find the following: Atom #p= #n= #e Ion #p= #n= #e= Isotope #p= #n #e 12 What are valence electrons? 13 Summarize what happens in a physical change, AND give an example of a physical change 14 Draw a box diagram showing a physical change. 15 Summarize what happens in a chemical change, AND give an example of a chemical change. 16 Draw a box diagram showing a chemical change 17 Summarize what happens in a nuclear reaction, AND give an example of a nuclear reaction 18 What is the mass defect? What is the mass converted into? 19 Explain fission and fusion AND give an example of each. Which (fusion or fission) releases more energy? 20 In Rutheford’s gold foil experiment, what 3 possible things happened to the particles when fired at the gold foil? What conclusions can be determined from this? 21 Draw a picture of Phosphorous, including p+, n0, and e- in the correct energy levels (be able to do this for all elements 1-20 on the periodic table) 22 Write the 2 kinds of symbols (nuclear and hyphen notation) for an isotope of Calcium that has a mass of 42. 23Compare and contrast ions, isotopes and atoms, as well as give examples. 24 Rubidium has two naturally occurring isotopes, 85Rb (84.9118 amu) and 87Rb (86.9092 amu). If Rubidium has an average atomic mass of 85.47 amu, what is the abundance of each isotope (in percent)? 25 What is the average mass of Lithium if 7.42% exists as Li-6 (6.015 amu) and 92.58% exists at Li-7 (7.016 amu). 26 What is the molar mass of the following: Na Zn CO2 27 Determine the mass of the following: 2.0 moles AuCl 3.1 moles BaF2 28 Determine the number of moles in the following: 72.3 g NaBr 4.1 x 103 atoms H20 29 Determine the number of atoms/molecules in the following: 4.7 moles FeCl2 192.6 g BeO MgCl2