SOLUTIONS
advertisement
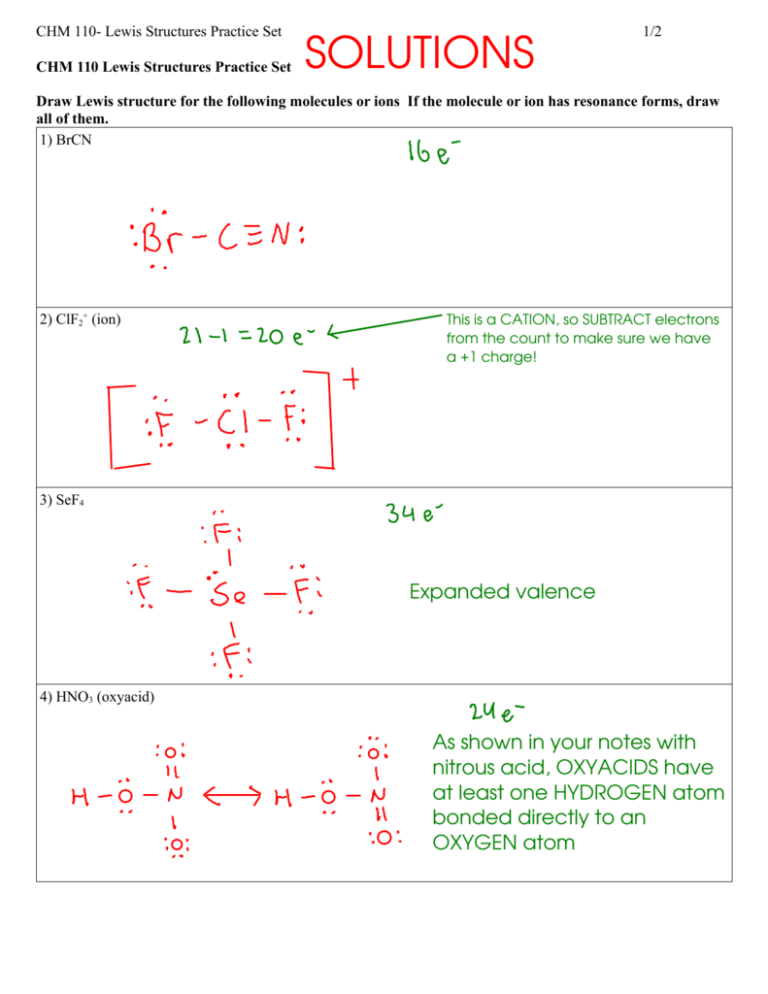
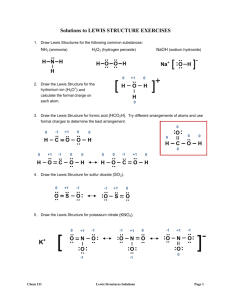
CHM 110- Lewis Structures Practice Set CHM 110 Lewis Structures Practice Set SOLUTIONS 1/2 Draw Lewis structure for the following molecules or ions If the molecule or ion has resonance forms, draw all of them. 1) BrCN 2) ClF2+ (ion) This is a CATION, so SUBTRACT electrons from the count to make sure we have a +1 charge! 3) SeF4 Expanded valence 4) HNO3 (oxyacid) As shown in your notes with nitrous acid, OXYACIDS have at least one HYDROGEN atom bonded directly to an OXYGEN atom CHM 110- Lewis Structures Practice Set 2/2 5) PBr3 6) NO2- (ion) This is an ANION, so ADD electrons to the count to make sure we have a -1 charge! 7) CH2Cl2 8) CH3COCH3 Take a hint from the way the chemical formula for this molecule was written ... there are three parts: ... which are chained together to make the mo;lecule













![[SO2]2[O2] [SO3]2 524.4K• 462.9K• 8.314 J mol•K • ln125.4 61.5K](http://s3.studylib.net/store/data/008432217_1-f6f0ddc631a0ec89f84a5e786b3339ef-300x300.png)