Document
advertisement
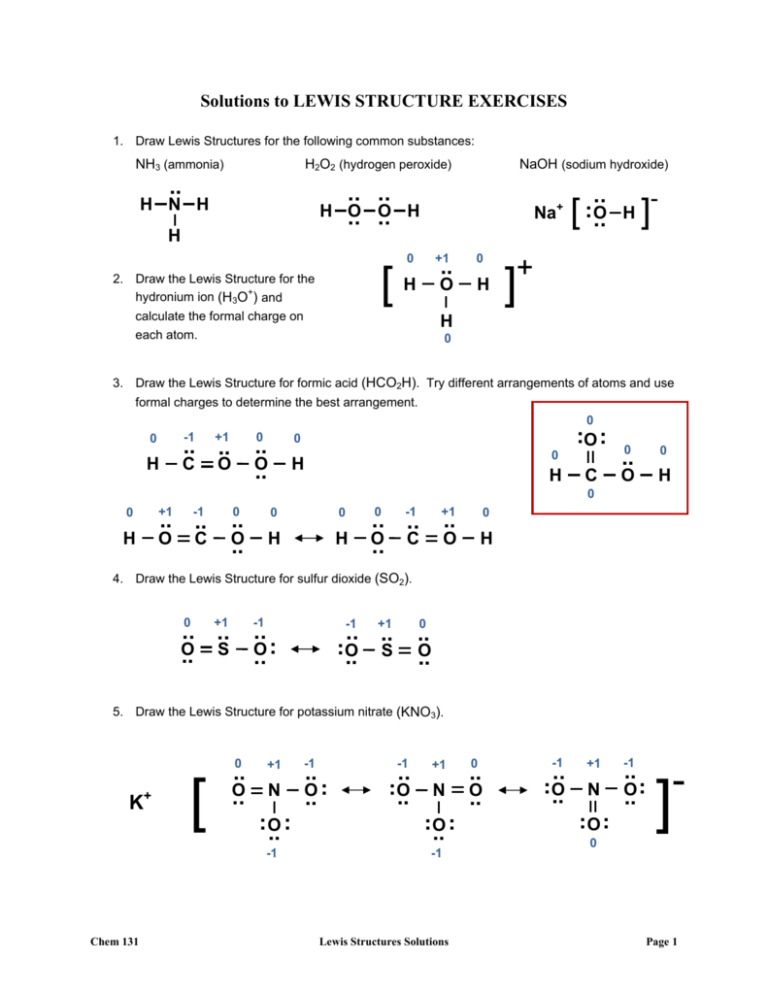
Solutions to LEWIS STRUCTURE EXERCISES 1. Draw Lewis Structures for the following common substances: NH3 (ammonia) H2O2 (hydrogen peroxide) .. H N H .. .. O .. O .. H H Na+ H 0 [ 2. Draw the Lewis Structure for the hydronium ion (H3O+) and NaOH (sodium hydroxide) +1 .. H O calculate the formal charge on each atom. 0 H ] .. .. O [ .. H ]- + H 0 3. Draw the Lewis Structure for formic acid (HCO2H). Try different arrangements of atoms and use formal charges to determine the best arrangement. 0 -1 +1 0 H C O 0 .. O .. .. .. ..O0 .. 0 H H C ..0 O 0 H 0 0 +1 -1 H O C 0 .. O .. .. .. 0 0 H H 0 -1 +1 0 O H .. .. .. O C .. 4. Draw the Lewis Structure for sulfur dioxide (SO2). 0 +1 -1 -1 .. . O. .. .. .. O .. S +1 0 .. .. .. O .. S .. O .. 5. Draw the Lewis Structure for potassium nitrate (KNO3). 0 K+ [ .. O .. +1 N .. O .. .. -1 Chem 131 -1 .. . O. .. -1 .. .. O .. +1 N .. O .. .. -1 Lewis Structures Solutions 0 .. O .. -1 .. .. O .. +1 -1 N O. .. O .. .. . .. - ] 0 Page 1 6. Draw the Lewis Structure for nitric acid (HNO3). Try attaching the H to either the N or an O. Which is the best arrangement? Compare the structure to the nitrate ion in the question above. 0 0 .. O .. The two structures at right both satisfy the octet rule and have reasonable formal charges. However, both involve O-O single bonds, which are very unstable (refer to bond energy table). H 0 N +1 -1 .. .. . O O. .. .. 0 .. O .. 0 .. N 0 0 .. .. O O .. .. 0 H The resonance structure below is the correct Lewis Structure for nitric acid. Note that it involves attaching an H+ to one of the O atoms in the nitrate ion (see problem 5 above). This H-O connection is characteristic of all oxyacids. 0 [ H .. O .. +1 N .. O .. .. 0 0 .. O .. H -1 7. Draw the Lewis Structure for chlorine trifluoride (ClF3). .. O .. +1 -1 N O. .. O .. .. . .. ] 0 .. .. .. . .. .. F. F Cl .. .. ..F .. .. 8. Draw the Lewis Structure for nitrous oxide (N2O). You should try different arrangements of atoms and different resonance structures. There are 5 different sets of Lewis Structures (some of these have equivalent resonance structures) you can come up with, stemming from two different provisional structures—one with O in the center, and the other with N in the center. You should find that the ones with O in the center are the least optimal. Of the structures with an N in the center, one is less optimal than the other two, which are nearly equivalent. One of these two is slightly more preferred than the other. We will revisit this problem in Expt 5 (Air Pollution ‘Lab’)… Chem 131 Lewis Structures Solutions Page 2




