בס"ד
advertisement

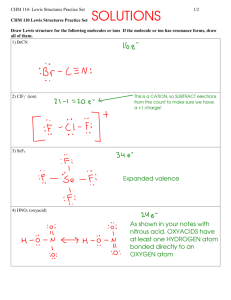
בס"ד 11 'תרגיל בית מס 34. Assign formal charges to the atoms in the following species, and then select the more likely skeletal structure. (a) (b) (c) (d) H2NOH or H2ONH SCS or CSS NFO or FNO SOCl2 or OSCl2 or OCl2S 41. Write a plausible Lewis structure for crotonaldehyde, CH3CHCHCHO, a substance used in tear gas and insecticides. 42. Write a plausible Lewis structure for C3O2, a substance known as carbon suboxide. 62. The molecular shape of BF3 (Table 11.1) is planar. If a fluoride ion is attached to the B atom of BF3 through a coordinate covalent bond, the ion BF4- results. What is the shape of the ion? 78. Write a Lewis structure of hydroxylamine molecule, H2NOH. Then, with data from Table 11.2, determine all the bond length. 88. A 0.325-g sample of a gaseous hydrocarbon occupies a volume of 193 ml at 749 mmHg and 26.1 ºC. Determine the molecular mass, and draw a plausible Lewis structure for this hydrocarbon.