Problem Solving with Ksp
advertisement
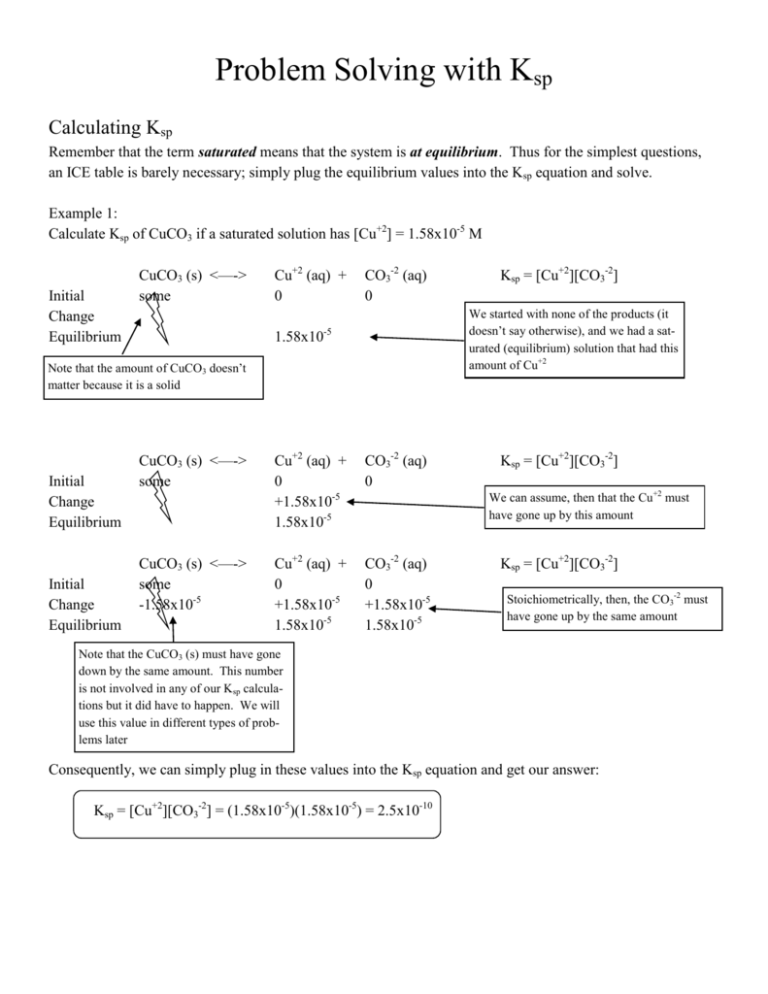
Problem Solving with Ksp Calculating Ksp Remember that the term saturated means that the system is at equilibrium. Thus for the simplest questions, an ICE table is barely necessary; simply plug the equilibrium values into the Ksp equation and solve. Example 1: Calculate Ksp of CuCO3 if a saturated solution has [Cu+2] = 1.58x10-5 M Initial Change Equilibrium CuCO3 (s) <—-> some Cu+2 (aq) + 0 CO3-2 (aq) 0 We started with none of the products (it doesn’t say otherwise), and we had a saturated (equilibrium) solution that had this amount of Cu+2 -5 1.58x10 Note that the amount of CuCO3 doesn’t matter because it is a solid Initial Change Equilibrium Initial Change Equilibrium Ksp = [Cu+2][CO3-2] CuCO3 (s) <—-> some Cu+2 (aq) + 0 +1.58x10-5 1.58x10-5 CO3-2 (aq) 0 CuCO3 (s) <—-> some -1.58x10-5 Cu+2 (aq) + 0 +1.58x10-5 1.58x10-5 CO3-2 (aq) 0 +1.58x10-5 1.58x10-5 Ksp = [Cu+2][CO3-2] We can assume, then that the Cu+2 must have gone up by this amount Ksp = [Cu+2][CO3-2] Stoichiometrically, then, the CO3-2 must have gone up by the same amount Note that the CuCO3 (s) must have gone down by the same amount. This number is not involved in any of our Ksp calculations but it did have to happen. We will use this value in different types of problems later Consequently, we can simply plug in these values into the Ksp equation and get our answer: Ksp = [Cu+2][CO3-2] = (1.58x10-5)(1.58x10-5) = 2.5x10-10 Example 2: Calculate Ksp of Ag2S if a saturated solution has [S-2] = 2.92x10-17 M Initial Change Equilibrium Ag2S (s) <—-> some -2.92x10-17 2 Ag+1 (aq) + S-2 (aq) 0 0 -17 +5.84x10 +2.92x10-17 5.84x10-17 2.92x10-17 Note that the amount of Ag2S doesn’t matter because it is a solid Ksp = [Ag+1]2[S-2] Note that the amount of Ag+1 is twice as much because of the stoichiometry Plug the equilibrium values in and calculate Ksp Ksp = [Ag+1]2[S-2] = (5.84x10-17)2(2.92x10-17) = 9.96x10-50 Example 3: Calculate the Ksp of Ca3(PO4)2 if a saturated solution has [Ca+2] = 2.14x10-6 Initial Change Equilibrium Ca3(PO4)2 (s) <—-> 3 Ca+2 (aq) + 2 PO4-3 (aq) some 0 0 -7 -6 -7.14x10 +2.14x10 +1.43x10-6 2.14x10-6 1.43x10-6 Note that the amount of Ca3(PO4)2 is only 1/3 of the amount of Ca+2 because of the stoichioimetry Ksp = [Ca+2]3[PO4-3]2 Note that the amount of PO4-3 is 2/3 of the Ca+2 because of the stoichiometry Ksp = [Ca+2]3[PO4-3]2 = (2.14x10-6)3(1.43x10-6)2 = 2.0x10-29 Calculating Solubility from Ksp Solubility is the amount of a solid that will dissolve to make a saturated solution. Consequently, if we know what the Ksp value of a substance is, we should be able to calculate how much of it will dissolve. There are, however, a couple of tricky points to this. The first of which is simply, where on the ICE chart is solubility? Since solubility is how much will dissolve, the number we are looking for is the change of ionic solid. This leads us to an interesting mathematical point. For example: Example 1 The Ksp of AgBr is 3.2x10-13. Calculate the molar solubility of AgBr. Initial Change Equilibrium AgBr (s) <—-> some Ag+1 (aq) + 0 Br-1 (aq) 0 Ksp = [Ag+1][Br-1] = 3.2x10-13 We start with some of the AgBr and none of the Ag+1 or Br-1 because the problem mentions no initial amounts How much of the AgBr will dissolve? We don’t know so we put “x” for that amount... Initial Change Equilibrium Initial Change Equilibrium Initial Change Equilibrium AgBr (s) <—-> some -x Ag+1 (aq) + 0 Br-1 (aq) 0 AgBr (s) <—-> some -x Ag+1 (aq) + 0 +x Br-1 (aq) 0 +x AgBr (s) <—-> some -x Ag+1 (aq) + 0 +x x Br-1 (aq) 0 +x x Ksp = [Ag+1][Br-1] = 3.2x10-13 An unknown amount of AgBr dissolves. This is the solubility. Ksp = [Ag+1][Br-1] = 3.2x10-13 We must then gain an stoichiometric amount of the products Ksp = [Ag+1][Br-1] = 3.2x10-13 At equilibrium we then have x for each of the products And here is where the tricky part comes into play. Mathematically, we only worry about the products in a Ksp calculation but what were are really interested in is the change of the AgBr. This is how much is dissolving so this is the solubility. Ksp = [Ag+1][Br-1] = 3.2x10-13 3.2x10-13 = x2 x = 5.66x10-7 moles/L In this simple case it turns out that the x we are looking for is the same as the products so it is very easy but that will not always be the case. Example 2 The Ksp of PbI2 is 8.7x10-9. Calculate the molar solubility of PbI2. Initial Change Equilibrium PbI2 (s) <—-> some -x Pb+2 (aq) + 0 +x x 2 I-1 (aq) 0 +2x 2x Ksp = [Pb+2][I-1]2 = 8.7x10-9 8.7x10-9 = (x)(2x)2 8.7x10-9 = 4x3 x = 1.30x10-3 moles/L Ksp = [Pb+2][I-1]2 = 8.7x10-9 Note that the I-1 has to increase by 2x because of the stoichiometry. This 2x is then squared because of the Ksp equation Again, though, the x we are looking for is the change (solubility) of the solid PbI2. It is a little goofy because the x we are solving for is not actually included in the Ksp equation itself. Example 3 The Ksp of Cu3(AsO4)2 is 7.6x10-36. Calculate the molar solubility of Cu3(AsO4)2 . Initial Change Equilibrium Cu3(AsO4)2 (s) <—-> 3 Cu+2 (aq) + 2 AsO4-3 (aq) some 0 0 -x +3x +2x 3x 2x Ksp = [Cu+2]3[AsO4-3]2 = 7.6x10-36 7.6x10-36 = (3x)3(2x)2 7.6x10-36 = 108x5 x = 3.71x10-8 moles/L Ksp = [Cu+2]3[AsO4-3]2 = 7.6x10-36 Note how each of the products must increase by their stoichiometric coefficients on the products side Because of problems like these, you must be careful when comparing Ksp values. Comparing Ksp Values If you are asked to compare the solubility of different substances by comparing their Ksp values, there are two situations that arise. The first is the very simple comparison of like substances. For example: Example 1: Which is more soluble, AgCl (Ksp = 1.8x10-10) or AgI (Ksp = 1.5x10-16) The short answer is that AgCl is more soluble because the Ksp value is larger. We can prove that by doing an ICE chart for each and comparing the x values. Initial Change Equilibrium Initial Change Equilibrium AgCl (s) <—-> some -x Ag+1 (aq) + 0 +x x Cl-1 (aq) 0 +x x Ksp = [Ag+1][Cl-1] = 1.8x10-10 1.8x10-10 = x2 x = 1.34x10-5 AgI (s) <—-> some -x Ag+1 (aq) + 0 +x x I-1 (aq) 0 +x x Ksp = [Ag+1][I-1] = 1.5x10-16 1.5x10-16 = x2 x = 1.22x10-8 We see that AgCl is more soluble as expected. However, you must be careful because the different mathematics seen on the previous pages can lead to different answers. For example: Example 2: Which is more soluble, AgCl (Ksp = 1.8x10-10) or Ag2CrO4 (Ksp = 9.0x10-12)? It would appear at first glance that AgCl is more soluble because the Ksp value is bigger. However, when you do the ICE charts you realize that they have very different mathematics which lead to a surprising result… Initial Change Equilibrium Initial Change Equilibrium AgCl (s) <—-> some -x Ag+1 (aq) + 0 +x x Cl-1 (aq) 0 +x x Ag2CrO4 (s) <—-> some -x 2 Ag+1 (aq) + CrO4-2 (aq) 0 0 +2x +x 2x x Ksp = [Ag+1][Cl-1] = 1.8x10-10 1.8x10-10 = x2 x = 1.34x10-5 Ksp = [Ag+1]2[CrO4-2] = 9.0x10-12 9.0x10-12 = (2x)2(x) 9.0x10-12 = 4x3 x = 1.31x10-4 We see that Ag2CrO4 is actually more soluble than AgCl even though AgCl has a Ksp value that is larger. This occurs because the AgCl get mathematically worked by x2 but the Ag2CrO4 gets worked by 4x3 because of the Ksp requirements. Consequently… Ksp values may only be directly compared if they have the same number of ionic pieces which will lead to the same Ksp mathematics Example 3: Put the following substances in order of least soluble to most soluble: Barium Carbonate BaCO3 5x10-9 Cadmium Sulfide CdS 8x10-28 Lead (II) sulfate PbSO4 6.3x10-7 Silver chloride AgCl 1.8x10-10 Since each of these substances break into only 2 pieces, their Ksp values can be directly compared to each other. Thus, put them in order of smallest to largest Ksp values: CdS (Ksp = 8x10-28) < AgCl (Ksp = 1.8x10-10) < BaCO3 (Ksp = 5x10-9) < PbSO4 (Ksp = 6.3x10-7) Example 4: Put the following substances in order of least soluble to most soluble: Copper (II) hydroxide Cu(OH)2 4.8x10-20 Silver sulfide Ag2S 6x10-51 Barium fluoride BaF2 1.7x10-6 Silver sulfate Ag2SO4 1.5x10-5 Since each of these substances break into 3 pieces, their Ksp values can be directly compared to each other. Thus, put them in order of smallest to largest Ksp values: Ag2S (Ksp = 6x10-51) < Cu(OH)2 (Ksp = 4.8x10-20) < BaF2 (Ksp = 1.7x10-6) < Ag2SO4 (Ksp = 1.5x10-5) Example 5: Put the following substances in order of least soluble to most soluble: Calcium fluoride CaF2 3.9x10-11 Copper (I) bromide CuBr 5.3x10-9 Lanthanum iodate La(IO3)3 7.4x10-14 Each of these substances breaks into a different number of pieces. Thus, each must be solved for individually and then compared. You cannot compare the Ksp values directly as above. Initial Change Equilibrium Initial Change Equilibrium Initial Change Equilibrium CaF2 (s) <—-> some -x Ca+2 (aq) + 0 +x x 2 F-1 (aq) 0 +2x 2x Ksp = [Ca+2][F-1]2 = 3.9x10-11 3.9x10-11= (x)(2x)2 3.9x10-11= 4x3 x = 2.14x10-4 CuBr (s) <—-> some -x Cu+1 (aq) + 0 +x x Br-1 (aq) 0 +x x Ksp = [Cu+1][Br-1] = 5.3x10-9 5.3x10-9= x2 x = 7.28x10-5 La(IO3)3 (s) <—-> some -x La+3 (aq) + 0 +x x 3 IO3-1 (aq) 0 +3x 3x Ksp = [La+3][IO3-1]3 = 7.4x10-14 7.4x10-14= (x)(3x)3 7.4x10-14= 27x4 x = 2.29x10-4 Comparing each of the values we see that the correct order is: CuBr (x = 7.28x10-5) < CaF2 (x = 2.14x10-4) < La(IO3)3 (x = 2.29x10-4) Calculating Solubility in Units other than Moles/Liter Every problem we’ve looked at so far has simply asked for the solubility. Since Ksp is a subset of Kc all of the answers are going to be in M units or moles/L because the equations are all in terms of concentration [x]. However, using some simple molar mass mathematics, we can transform these answers into other units. Mass/Liter Example 1: Calculate the solubility of Zn(OH)2 in milligrams per Liter (mg/L). Ksp = 4.5x10-17 To do this, simply solve the problem as before: Initial Change Equilibrium Zn(OH)2 (s) <—-> some -x Zn+2 (aq) + 0 +x x 2 OH-1 (aq) 0 +2x 2x Ksp = [Zn+2][OH-1]2 = 4.5x10-17 4.5x10-17 = (x)(2x)2 4.5x10-17 = 4x3 x = 2.24x10-6 The solubility of Zn(OH)2 is 2.24x10-6 moles/L. But we also know there are 1000 mg in a gram and we know from the Periodic Table that the molar mass of Zn(OH)2 is 99.4 grams/mole. Thus: 2.24 x10-6 moles x 99.4 grams x 1000 mg = 0.223 mg 1L mole g L Example 2: Calculate the solubility of BaSO4 in milligrams per Liter (mg/L). Ksp = 1.1x10-10 Initial Change Equilibrium 1000 mg = 1 g BaSO4 (s) <—-> some -x Ba+2 (aq) + 0 +x x SO4-2 (aq) 0 +x x BaSO4: 233.4 g/mole 1.05 x10-5 moles x 233.4grams x 1000 mg = 2.45 mg 1L mole g L Ksp = [Ba+2][SO4-2] = 1.1x10-10 1.1x10-10= (x)(x) 1.1x10-10 = x2 x = 1.05x10-5 Mass If we are given the volume, we can actually determine how many grams of a substance dissolves or how many grams are left once a substance dissolves. Example 1: How many mg of Pb+2 ions are in 250 mL of a saturated solution of PbCO3? Ksp = 7.4x10-14 Initial Change Equilibrium PbCO3 (s) <—-> some -x Pb+2 (aq) + 0 +x x CO3-2 (aq) 0 +x x Ksp = [Pb+2][CO3-2] = 7.4x10-14 7.4x10-14= (x)(x) 7.4x10-14= x2 x = 2.72x10-7 The solubility of PbCO3 is 2.72x10-7 moles/L. But we also know there are 1000 mg in a gram and we know from the Periodic Table that the molar mass of Pb+2 is 207.2 grams/mole. We also know that there are 1000 mL in a Liter and there are 250 mL. Thus: 2.72 x10-7 moles x 207.2 grams x 1L x 1L mole 1000 mL 1000 mg x 250 mL = 0.014 mg Pb+2 g 1 Example 2: If 5 grams of Ca(OH)2 are put into 2 L of water and the solution becomes saturated, how many grams of Ca(OH)2 are left undissolved? Ksp = 6.5x10-6 Initial Change Equilibrium Ca(OH)2 (s) <—-> some -x Ca+2 (aq) + 0 +x x 2 OH-1 (aq) 0 +2x 2x Ksp = [Ca+2][OH-1]2 = 6.5x10-6 6.5x10-6 = (x)(2x)2 6.5x10-6 = 4x3 x = 1.18x10-2 The solubility of Ca(OH)2 is 1.18x10-2 moles/L. But we also know there are 74.1 g/mole for Ca(OH)2. 1.18 x10-2 moles x 74.1 grams x 2 L = 1.75 grams Ca(OH)2 dissolved 1L mole 1 Thus there are 5 grams—1.75 grams dissolved = 3.25 grams left undissolved.








