ME 354 Tutorial #2 – Availability

ME 354 Tutorial #5 – Otto Cycle Winter 2001
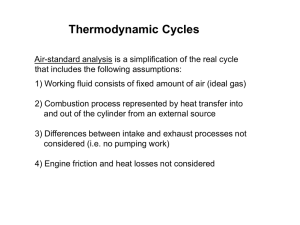
An ideal Otto cycle has a compression ratio of 8. At the beginning of the compression process air is at 95 kPa and 27 C. 750 kJ/kg of heat is transferred to the air during the constant volume heat-addition process. Assume constant specific heats at room temperature. Determine: a) The pressure and temperature at the end of the heat-addition process b) The net work output c) The thermal efficiency of the cycle
Step 1: Draw a process diagram to represent the system
To better visualize what is happening during the cycle we can draw a P-v process diagram.
Step 2: Prepare a property table
1
2
3
T (K)
300
4
Step 3: State your assumptions
Assumptions:
1) ke, pe 0
2) air-standard assumption are applicable
P (kPa)
95
3) air is an ideal gas with constant specific heats
Step 4: Calculations
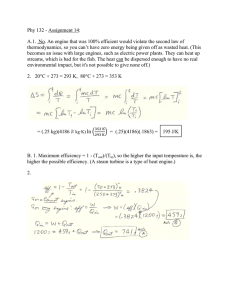
Part a)
In order to find the pressure and temperature at state 3 we need to find the temperature and pressure of an adjacent state (2 or 3) so we can use some relation between that state and state 3. We will find P
2
and T
2
.
Referring to our process diagram, we see that from state 1 to 2 we have an isentropic compression process. Referring to our ideal gas relations for isentropic processes, the ratio of the temperatures of the two states are related to the ratio of their specific volumes by Eq1.
T
2
T
1
v
1 v
2
k
1
(Eq1)
Noting that the ratio v
1
/v
2
(equivalent to V max
/V min
) is the compression ratio, r, and the value of k for air is 1.4, we can solve for the temperature at state 2.
T
2
T
1
v
1 v
2
k
1
( 300 )
1 .
4
1 = 689.2 K
Writing the 1 st law for the constant heat-addition process from state 2 to 3 we can relate state 2 to state 3 as seen in Equation 2. Note: we have used the assumption that ke & pe are approximately zero here. q in
u
3
u
2
(Eq2)
For an ideal gas with constant specific heats we have a relation for the change in internal energy in terms of the change in temperature as shown in Eq3. u
3
u
2
c v
T
3
T
2
(Eq3)
Since we are given the value of q in
in the problem statement we can substitute
Eq3 into Eq2 and rearrange to solve for the temperature at state 3.
q in
c v
( T
3
T
2
)
T
3
q in c v
T
2
750
kJ kg
0 .
718
kg kJ
K
689 .
2 K
= 1733.8K answer a)
To find the pressure at state 2 we can use the ideal gas law as shown in Eq4.
R
P
1 v
1
P
2 v
2
P
2
P
1
v
1
T
2
(Eq4)
T
1
T
2 v
2
T
1
Noting again, that v
1
/v
2
is the compression ratio we can solve for the pressure at state 2.
P
2
P
1
v v
2
1
T
2
T
1
( 95 [ kPa ])( 8 )
689 .
2 [
300 [ K
K
]
]
Using the ideal gas to relate state 3 to state 2 we obtain Eq5.
R
P
3
T
3 v
3
P
2
T
2 v
2
P
3
P
2
v v
2
3
T
T
3
2
=1746 kPa
(Eq5)
Noting that v
2
= v
3
, we can solve for the pressure at state 3.
P
3
P
2
v v
2
3
T
T
3
2
( 1746 [ kPa ])
1733 .
8 K
689 .
2 K
= 4392.4kPa
answer a)
Part b)
To find an expression for the net work output we can perform an overall energy balance on the cycle as shown in Eq6. q in
w in
q out
w out
w net
w out
w in
q in
q out
(Eq6)
We are given q in
in the problem statement so the problem reduces to finding q out
. Writing a first law balance (similar to what we did for q in
) we can find q out
in terms of the temperature difference between state 4 and 1as shown in Eq7.
q out
u
4
u
1
c v
T
4
T
1
(Eq7)
To find the temperature at state 4 we make use of the fact that the expansion from state 3 to state 4 is isentropic so we can use the relation shown in Eq1 again.
T
4
T
3
v
3 v
4
k
1
(Eq8)
Noting that v
3
/v
4
is the inverse of the compression ratio we can determine the temperature at state 4.
T
4
1 .
4
1
( 1733 .
8 [ K ])
1
8
754.7 K
Substituting this result into Eq7 we can determine q out
. q out
c v
T
4
T
1
0 .
718
kg kJ
K
754 .
7 [ K ]
300 [ K ]
326.5 kJ/kg
Using this result with the given q in
=750kJ/kg and Eq6 we can solve for the net work output. w net
q in
q out
( 750
326 .
5 )
kJ kg
423.5 kJ/kg answer b)
Part c)
To calculate the thermal efficiency we can use our general expression
(benefit/cost).
th
benefit cos t
w net
423 .
750
5
0.565 answer c) q in
We could have also used the expression for the Otto cycle thermal efficiency in terms of the compression ratio.
th
1
1 k
1
1
1
0.565 answer c) r 8 0 .
4
Step 5: Concluding Remarks & Discussion a) The pressure and temperature at the end of the heat-addition process were found to be 4392.4kPa and 1733.8 K respectively. b) The net work output was found to be 423.5 kJ/kg. c) The thermal efficiency of the cycle was found to be 0.565.