exam 1 could look roughly like this:
advertisement

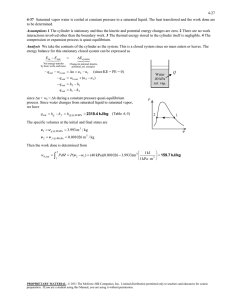
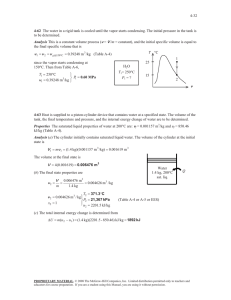
exam 1 could look roughly like this: exam 1 could look roughly like this: “exam 1 practice set 2” 3-51 4-29 solutions 3-51 A rigid tank that is filled with saturated liquid-vapor mixture is heated. The temperature at which the liquid in the tank is completely vaporized is to be determined, and the T-v diagram is to be drawn. Analysis This is a constant volume process (v = V /m = constant), and the specific volume is determined to be H2O v V m 2.5 m 3 0.1667 m 3 /kg 15 kg 75C When the liquid is completely vaporized the tank will contain saturated vapor only. Thus, T 2 v 2 v g 0.1667 m /kg 3 1 The temperature at this point is the temperature that corresponds to this vg value, T Tsat@v 0.1667 m3 /kg 187.0C (Table A-4) g v 4-29 Saturated water vapor is isothermally condensed to a saturated liquid in a pistoncylinder device. The heat transfer and the work done are to be determined. Assumptions 1 The cylinder is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions involved other than the boundary work. 3 The thermal energy stored in the cylinder itself is negligible. 4 The compression or expansion process is quasi-equilibrium. Analysis We take the contents of the cylinder as the system. This is a closed system since no mass enters or leaves. The energy balance for this stationary closed system can be expressed as E E inout Net energy transfer by heat, work, and mass E system Change in internal,kinetic, potential,etc. energies Wb,in Qout U m(u 2 u1 ) (since KE = PE = 0) Qout Wb,in m(u 2 u1 ) The properties at the initial and final states are (Table A-4) Water 200C sat. vapor Heat T1 20 0C v 1 v g 0.12721 m 3 / kg x1 1 u1 u g 2594 .2 kJ/kg P1 P2 1554 .9 kPa T2 20 0C v 2 v f 0.001157 m 3 / kg x2 0 u 2 u f 850 .46 kJ/kg T 2 The work done during this process is wb,out 2 1 1 kJ PdV P(v 2 v 1 ) (1554.9 kPa)(0.001 157 0.12721) m 3 /kg 1 kPa m 3 That is, wb,in 196.0 kJ/kg Substituting the energy balance equation, we get qout wb,in (u 2 u1 ) wb,in u fg 196.0 1743.7 1940kJ/kg 1 v 196 .0 kJ/kg