Phy 132 - Assignment 14:
advertisement

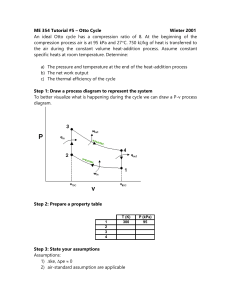
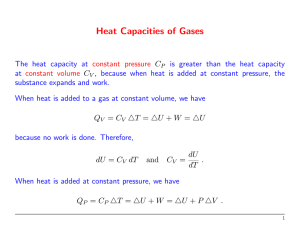
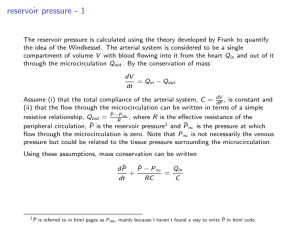
Phy 132 - Assignment 14: A.1. No. An engine that was 100% efficient would violate the second law of thermodynamics, so you can’t have zero energy being given off as wasted heat. (This becomes an issue with large engines, such as electric power plants. They can heat up streams, which is bad for the fish. The heat can be dispersed enough to have no real environmental impact, but it's not possible to give none off.) 2. 20°C + 273 = 293 K, 80°C + 273 = 353 K = (.25 kg)(4186 J/ kg·K) ( ) = (.25)(4186)(.1863) = 195 J/K B. 1. Maximum efficiency = 1 - (Tout)/(Tin), so the higher the input temperature is, the higher the possible efficiency. (A steam turbine is a type of heat engine.) 2. C. D.1. No. A refrigerator works by pumping heat from its inside to its outside. Having the door open just lets it flow back in again. That doesn't remove heat from the room. 2. E. The formulas from sec. 13 give the work done on the gas. If you use them on an engine, the work comes out negative, so this question asks for the work done by the gas. That’s why I’ve used the negative of those formulas. b). Qin = W + Qout = 4.05 + 10.0 = 14.05 kJ e = W/Qin = 4.05/14.05 = .288 (or 28.8%) F. Start with part b: Qin = W + Qout 3200 = W + 2600 W = 600 J a. e = W/Qin = (600 J)/(3200) = .1875 c. Power = W/t = (600 J)/(.3 s) = 2000 W d. Carnot efficiency = 1 Tout 383 1 = 1 - .732 = .268 Tin 523 110 + 273 = 383 K, 250 + 273 = 523 K