Draw Lewis Structure- Show All-Lone Pairs
advertisement

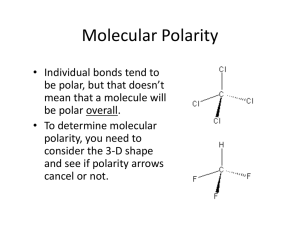
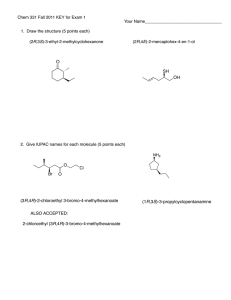
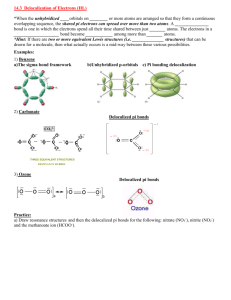
Bonding Review Assignment Due Friday, March 18, 2014 CHEM 163, Spring 2014 BR #11 For each species listed: 1) Draw a Lewis Structure- show all-lone pairs, include all resonance structures (if none, write, NR). 2) List the Formal Charge on each atom in each resonance structure. Circle the “best” resonance structure. 3) List the Oxidation State of each atom in each resonance structure. 4) State the VSEPR Electron Group Geometry (EGG) of the central atom (# of regions of electron density = # of clouds ≠ # of bonds. For non-single center molecules or ions give the EGG for all hub atoms. Use the “best” resonance structure. 5) State the VSEPR Molecular Group Geometry (MGG) or Actual Geometry. For non-single center molecules or ions give the MGG for all hub atoms. Use the “best” resonance structure. 6) Determine the Polarity of each Bond-Draw dipole moments aside each bond. 7) Determine the Polarity of the Species using information from 5 and 6. Polar and Non-polar are sufficient descriptions. 8) List the Hybridization of each atom in the “best” resonance structure. 9) Give the Valence Bond Theory Description of Bonding for the “best” resonance structure. Sketch a picture using hybrid orbitals. Label all and bonds. Cl2O2, with the chlorines as central hubs. [N2F]+, with this ion add a sketch using only atomic orbitals (no hybrids) SO3NH2- (sulfamate) XeF2