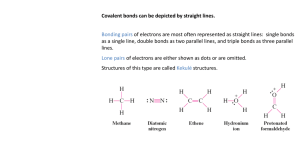
Molecular Polarity
advertisement
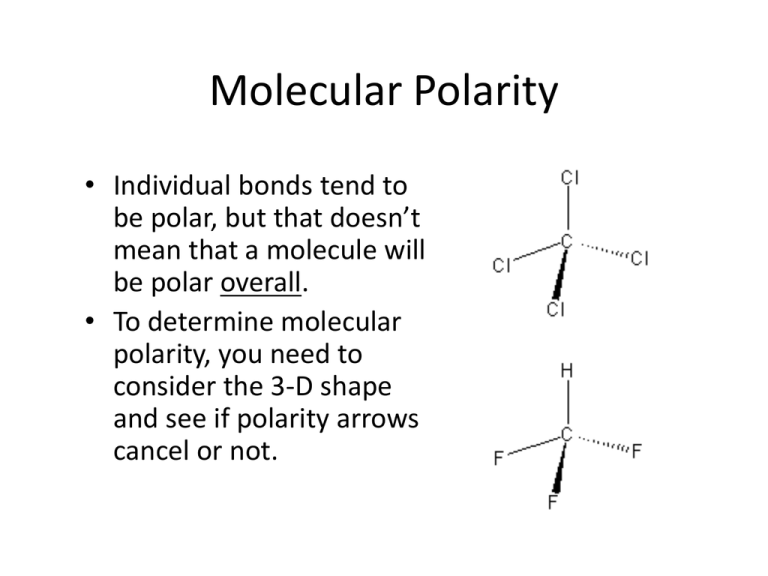
Molecular Polarity • Individual bonds tend to be polar, but that doesn’t mean that a molecule will be polar overall. • To determine molecular polarity, you need to consider the 3-D shape and see if polarity arrows cancel or not. Note that all these molecules have polar bonds. Two of these molecules are nonpolar because their symmetry causes the charges to cancel out: Copyright by the Glencoe Division of Macmillan/McGraw-Hill School Publishing Company Multiple Valid Lewis Structures • Sometimes more than one Lewis structure can be drawn for the same molecule. • For example, ozone (O3). Resonance Forms • Resonance forms are also known as resonance structures. • Resonance forms have the same relative placement of atoms, but different locations of bonding and lone e- pairs. Resonance Hybrid • Neither resonance form is a true picture of the molecule. • The molecule exists as a resonance hybrid, which is an average of all resonance forms. • In a resonance hybrid, e- are delocalized over the entire molecule. Important Resonance Forms • If all resonance forms have the same surrounding atoms, then each contributes equally to the resonance hybrid. • If this is not the case, then one or more resonance forms will dominate the resonance hybrid. • How can we determine which forms will dominate? Exceptions to the Octet Rule • We’ve already discussed expanded valence cases, but there are other exceptions as well. e- deficient atoms like Be and B, e.g. BeCl2 and BF3. Compounds w/ odd # of e-’s: free radicals. Examples include NO and NO2. Expanded valence – when d orbitals are used to accommodate more than an octet.