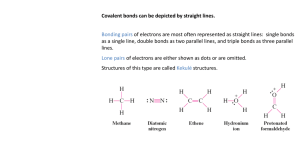
IB Chemistry: Delocalization
advertisement
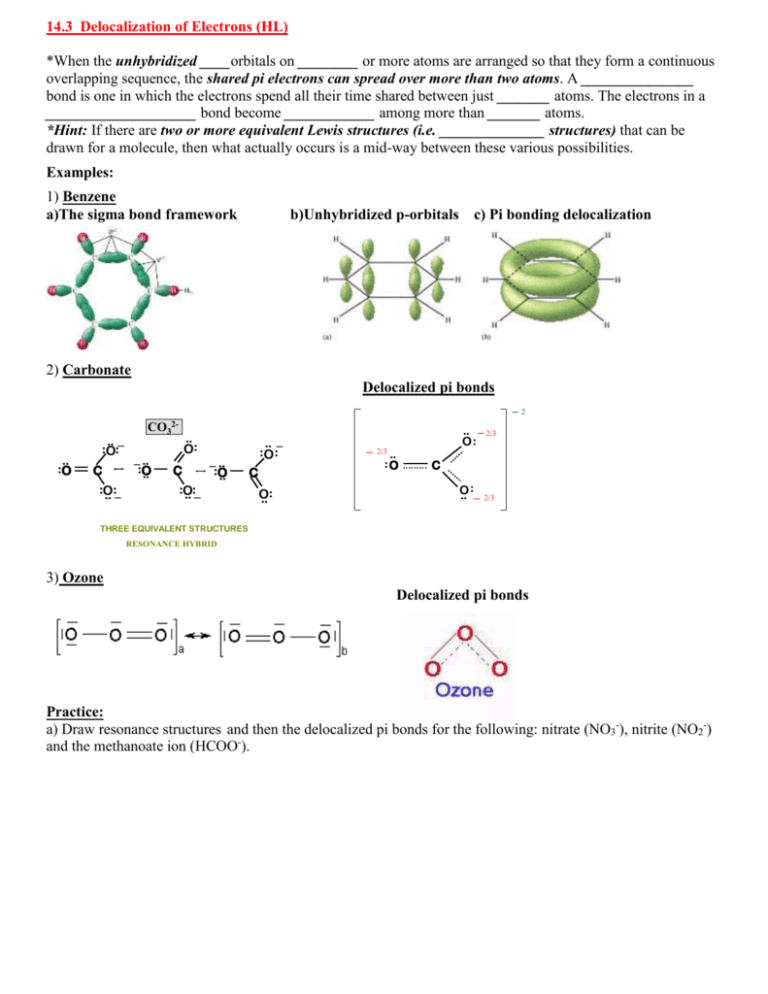
14.3 Delocalization of Electrons (HL) *When the unhybridized ____orbitals on ________ or more atoms are arranged so that they form a continuous overlapping sequence, the shared pi electrons can spread over more than two atoms. A _______________ bond is one in which the electrons spend all their time shared between just _______ atoms. The electrons in a ____________________ bond become ____________ among more than _______ atoms. *Hint: If there are two or more equivalent Lewis structures (i.e. ______________ structures) that can be drawn for a molecule, then what actually occurs is a mid-way between these various possibilities. Examples: 1) Benzene a)The sigma bond framework b)Unhybridized p-orbitals c) Pi bonding delocalization 2) Carbonate Summary Structurepi forbonds Carbonate Ion Delocalized Resonance in the Carbonate Ion .. :O .. _ :O: C :O .. :_ CO32_ .. :O .. .. O: C :O .. :_ _ .. :O .. _2 .. _ :O : C O .. : _ .. _ 2/3 O: 2/3 .. :O C : O .. _ 2/3 THREE EQUIVALENT STRUCTURES RESONANCE HYBRID 3) Ozone Delocalized pi bonds Practice: a) Draw resonance structures and then the delocalized pi bonds for the following: nitrate (NO3-), nitrite (NO2-) and the methanoate ion (HCOO-). 14.3 Practice: Resonance and Delocalization Resonance Structure 1 Resonance Structure 2 Resonance Structure 3 Delocalized Structure nitrate ion carbonate ion Resonance Structure 1 Resonance Structure 2 Delocalized Structure nitrite ion ozone benzene carboxylate ion More practice Draw all resonance structures for: SO2, SO3, SCN-