Using titration curves to determine pKa values
advertisement

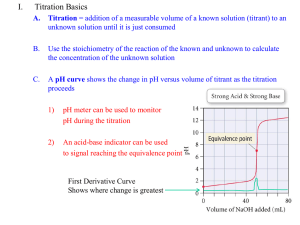
Titration curve and pKa value Using titration curves to determine pKa values The pH value where the titratable group is half-protonated is equal to the pKa if the titration curve follows the Henderson-Hasselbalch equation. Most pKa calculation methods silently assume that all titration curves are Henderson-Hasselbalch shaped, and pKa values in pKa calculation programs are therefore often determined in this way. In chemistry, the Henderson-Hasselbalch equation describes the derivation of pH as a measure of acidity (using pKa, the acid dissociation constant) in biological and chemical systems. The equation is also useful for estimating the pH of a buffer solution and finding the equilibrium pH in acid-base reactions It is widely used to calculate isoelectric point of the proteins. The general equations for acid-base reactions and the basic theory behind pKa calculations. The basic equations for acid-base reactions The dissociation of a proton from an acid generally takes the form HA + H2O -> H3O+ + A- (Eq. 4.1) The free energy change for this reaction (DGa) can be related to an equilibrium constant (Ka) for the reaction (we exclude water and replace H3O+ with H+ for simplicity): For acid-base reactions it is customary to report the pKa values, which is simply –log(Ka). The Henderson-Hasselbalch equation is a rearrangement of equation From the Equation,. it is seen that the pKa value of an acid is the pH value where the concentrations of the protonated and deprotonated forms of the acid are present at the exact same concentrations. Furthermore by rearranging Eq. and plotting fHA as a function of pH we get the well-known sigmoid titration curve The titration of an acid with a pKa value of 5.0 For the association of a proton with a base, a similar set of equations can be produced. The major difference being that the base reaction is: B + H3O+ -> BH+ + H2O The corresponding equilibrium constant thus becomes Calculating titration curves We now know the energy of every possible protonation state of a protein at a given pH value, and the next step is the conversion of these energies into fractional charges at each pH value for each residue in order to get the titration curves. A straight-forward way to find the occupancy of the different states in Table 4.1 is to evaluate the Boltzmann sum for each state. Here pi is the fraction of molecules in state i. Ei is the energy of state i, and the sum in the denominator is over all possible states of the system. k is Boltzmann’s constant and T is the temperature in Kelvin. The fractional charge of a particular group is simply the sum of the pi’s for all the states where the group is charged. Thus for group 1 in Table 4.1, for example, the charge is the sum of p1, p2, p3 and p4. From the calculated titration curves the pKa value for each group is determined as the pH where the group is half-protonated. This gives an accurate result only if the titration curve follows a HendersonHasselbalch shape. This is the case for most groups, but especially in active sites it is quite common to find groups that have very irregular titration curves. In these cases manual inspection of the titration curves is necessary in order to obtain meaningful results. Significance of pKa calculations Tell you which pKa values are highly shifted, and their approximate value. Give you the overall shape of the pH stability curve