Second Major Exam Philadelphia University 0510113 Analytical
advertisement

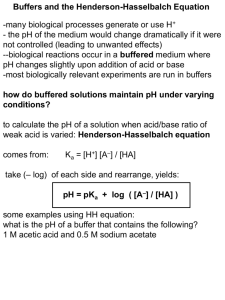
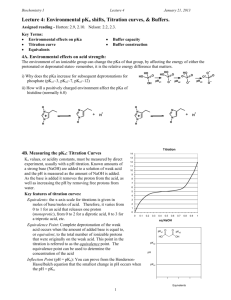
Second Major Exam Philadelphia University 0510113 Analytical chemistry Faculty of Pharmacy Sections: 1 to 6 Second semester 2014/2015 (10 May 2015) Dr. Ibrahim andDr. Zaher Name: ………………………………….. ID #:…………………… Sec.:……….. _____________________________________________________________________ 1. This examination paper comprises 8 questions in 4 pages, totalling 20 marks. 2. The overall marks of questions are shown in round brackets. 3. You should write your answers clearly Q1. Choose the best answer for the following questions.(5 marks) 1. The basic value on the pH scale is a. less than 7 b. greater than 7 c. equal to 7 d. equal to 8.8 2. Which of the following describes the Henderson Hasselbalch equation where HA is the weak acid? a. pH = pKa + log [A-]/[HA] b. pH = pKa- log [A-]/[HA] c. pH = pKa + log [HA]/[A-] d. pKa = pH + log [A-]/[HA] 3. Concerning buffers, which of the following is true? a. strong acid and bases are good buffers b. buffers may be a mixture of a weak acid and its conjugate base c. any weak acids and bases are making good buffers d. buffers may be a mixture of a weak acid and its conjugate acid 1 4. The pH 0.0003M HCl is a. b. c. d. 3.50 3.52 2.52 2.50 5. The pOH of 0.01 HCl is a. b. c. d. 2.00 12.00 1.00 13.00 Q2. Calculate the H+ for solution pH=1.9 ? (1marks) PH = 1.9 H+ =0.01258 Q3. Calculate the pH of a 0.01 M solution of ammonia? Kb=1.75X10-5(1 marks) Kb = 1.75x 10-5 = X2/.0.01 X = OH =4.18x104POH = 3.37 PH = 10.62 Q4. Calculate the pH for 1.4X10-2M sodium acetate? Ka= 1.75X10-5(1mark) Kb = 1x10-14 /1.75x10-5 = X2/1.4X10-2 X = OH =2.8x10-6 POH = 5.548 PH = 8.44 Q5.What is the weight of the CHCl2COOH (M.wt = 129 g /mol.) must be mixed with 15 mL of 5.0 M CHCl2COONa to make the pH of the solution=2.7?(Ka=5.0X10-2) (2 marks) PH = PKa + log A/HA 2.7 = -log 5x10-2 + log 15x5/mmol Mmole = 2.95 Mass = 2.95 x 129 = 380.7 gm 2 Q6.Draw the titration curve for the titration of 50 ml of 0.1M HF(Ka = 7.2x10-4) (4marks) versus 0.1MNaOH and show the following a) The value of equivalent point = more than 7 b) The buffer area in the curve c) The volume of NaOH used to reach equivalent point = 50 ml d) The pH at the med point of titration more than 7 Q7. Calculate the pH of solution that results when 40 ml of 0.1M CH3COOH (Ka = 1.75*10-5) are (4 marks) a) Diluted to 100 with distilled water. b) Mixed with 20 ml of 0.01M NaOH. A)400x 0.1 = 100 x M2 M2 = 0.04M 1.75 X10-5 = X2/0.04 X = H =0.000836 PH = 3.07 B)mmole of acid 40 x 0.1 =4 Mmole of base = 20 x 0.01 0.20 Excess acid 4 – 0.2 = 3.8 PH = 4.75 + log 0.2/ 3.8 = 3.47 3 Q8. Fictitious acid (H3Fi) is a triprotic acid with Ka values of precisely 4-, 7, and 10-Calculate the theoretical pH of the following solutions, given Kw = 1.0X10-14? (2 marks) (a) 0.15 M trisodium fictate, Na3Fi. (b) 0.15 M disodium fictate, Na2HFi. H3Fi ===H2Fi- + H+ pKa = 4 H2Fi-===HFi-2 + H+ pKa =7 HFi-2 ===Fi-3 +H+ + pKa =10 A)Kb = 1x10-14 /1x10-10 =X2/0.15 X = OH POH 2.41 PH 11.588 B) H+= the square root of 1x10-7 x 10-10 = 3x10-9 PH = 8.5 4 5