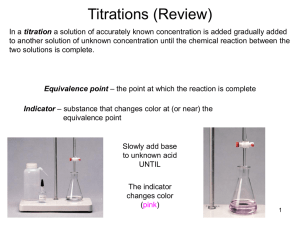
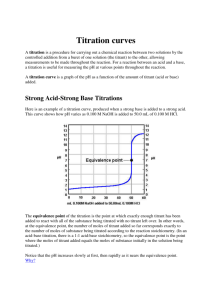
Titrations of Acids and Bases
advertisement
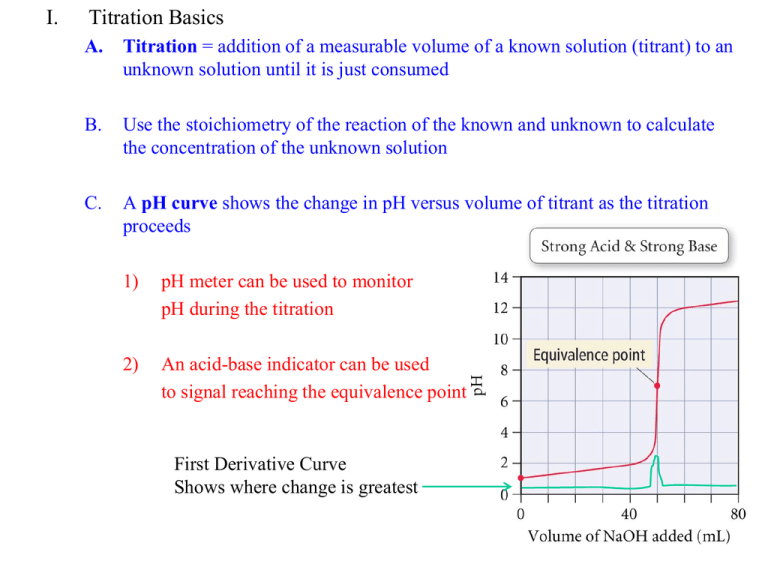
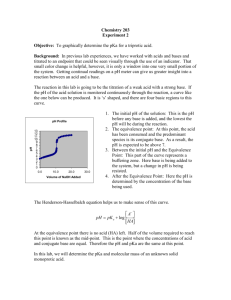
I. Titration Basics A. Titration = addition of a measurable volume of a known solution (titrant) to an unknown solution until it is just consumed B. Use the stoichiometry of the reaction of the known and unknown to calculate the concentration of the unknown solution C. A pH curve shows the change in pH versus volume of titrant as the titration proceeds 1) pH meter can be used to monitor pH during the titration 2) An acid-base indicator can be used to signal reaching the equivalence point First Derivative Curve Shows where change is greatest 3) Important points: a) pH increases slowly far from the equivalence point b) pH changes quickly near the equivalence point c) The equivalence point of a strong acid—strong base titration = 7.00 4) The titration of a strong base with a strong acid is almost identical II. Titration of a Weak Acid with a Strong Base A. B. Addition of a strong base to a weak acid forms a Buffer Solution 1) HA + OHA- + H2O 2) If not enough base has been added to complete the reaction: HA/A- buffer Important Points 1) pH increases more rapidly at the start than for a strong acid 2) pH levels off near pKa due to HA/A- buffering effect pH = pKa + log([A-]/[HA]) = pKa + log(1) = pKa (when [A-] = [HA]) 3) Curve is steepest near equivalence point. Equivalence Point > 7.0 4) Curve is similar to strong acid—strong base after eq. pt. where OH- is major ½ Equivalence Pt. pH = pKa 12.5 III. Titration of a Weak Base with a Strong Acid A. Similar problem to the titration of a weak acid with a strong base 1) Determine major species from the stoichiometry 2) Calculate pH from weak acid, buffer, or weak base accordingly B. Example: Titrate 100 ml of 0.10 M NH3 (Kb = 1.8 x 10-5) with 0.1 M HCl. IV. Titrations of Polyprotic Acids and Bases 1. Multiple Inflection Points = Multiple Equivalence Points will be seen 2. The volume required to reach each equivalence point will be the same CO32- + H+ HCO3Kb1 = KW/Ka2 = 1.8 x 10-4 HCO3- + H+ H2CO3 Kb2 = KW/Ka1 = 2.3 x 10-8 pKb1 pKb2 ½ Eq. pt 1 Eq. pt 1 ½ Eq. pt 2 Eq. pt 2 IV. Acid-Base Indicators A. Finding the equivalence point of a titration 1) Use a pH meter a) Plot pH versus titrant volume b) Center vertical region = equivalence point 2) Use an Acid-Base Indicator a) Acid-Base Indicator = molecule that changes color based on pH b) Choose an indicator that changes color at the equivalence point c) End Point = when the indicator changes color. If you have chosen the wrong indicator, the end point will be different than the eq. pt. d) Indicators are often Weak Acids that lose a proton (causing the color change) when [OH-] reaches a certain concentration HIn + OHIn- + H2O B. We can use the Henderson-Hasselbalch equation on Indicators as well 1) pH = pKa + log([In-]/[HIn]) 2) pH = pKa + log(1/10) for a color change a) log(1/10) = -1 b) pH for color change starting in acid is always pKa – 1 for any Indicator 3) For a basic solution titrated with acid, [In-]/[HIn] = 10/1 for color change a) Log(10/1) = +1, pH for color change will equal pKa + 1 b) Useful range for a pH Indicator is always pKa +/- 1 V. Experimental Details A. Three Titrations Today 1) Titrate 10 ml of unknown HCl with NaOH, using Methyl Red 2) Titrate 10 ml of unknown HOAc with NaOH, using Phenolphthalein 3) Titrate 25 ml of unknown Na2CO3 with HCl, using Methyl Orange 4) RECORD pH at which Indicator Changes Color B. We will go over starting, standardizing, and saving data on Palms in Lab C. Plotting your data--Example 1) Open txt file from LabQuest in Excel—it is TAB DELIMITED 2) Select all three columns of data—Excel will plot the pH curve and derivative 3) Insert XY Scatter plot with a smooth curve 4) Use the Derivative Curve to find the equivalence point 5) For weak acids/bases, use ½ volume from the equivalence point to find pKa D. Use your titration curves to answer all questions in the lab handout 1) Turn in a graph for each titration 2) Don’t worry about “Data Sheet” (manual titration) except for indicator color changes