Titrimetric Analysis of an Amino Acid
advertisement

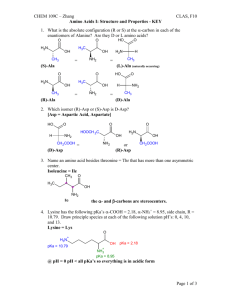
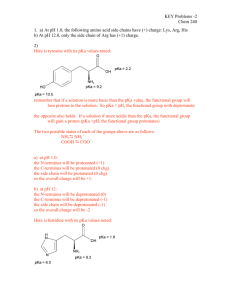
TITRIMETRIC ANALYSIS OF AN AMINO ACID Purpose: The purpose of this laboratory activity is to construct and study the titration curve of an unknown amino acid. Discussion: Answer these questions: 1. How is a titration curve constructed? 2. How do the titration curves of amino acids with non-ionizable side chains compare to the titration curves of amino acids with ionizable side chains? Draw example titration curves. 3. How will the titration curve be used to determine the pKa values and identify the unknown amino acid? Objectives: In this lab, you will… 1. develop a procedure to help construct a titration curve for the unknown amino acid. 2. determine the pKa of each proton of the amino acid. 3. compare pKa values to accepted pKa values for the amino acid. Materials: Unknown amino acids (AAUK1-7) 0.300 M KOH Universal indicator Procedures: 1. Develop your procedure and get it approved. 2. Run the experiment on lab day. 3. Hand in both a notebook and a full report one week after the first day you attempt the experiment. Analysis/Calculations/Error: 1. Be sure to save and print your titration curve. 2. Determine the pKa of each proton of the amino acid. 3. Compare pKa values to accepted pKa values for the amino acid.