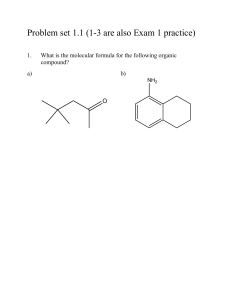
1) draw a skeleton structure for the molecule or ion, joining atoms by

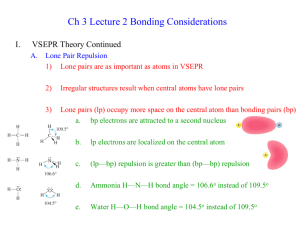
V alence S hell E lectron P air R epulsion T heory ( VSEPR ) is a model for determining molecular geometry which relies on one simple principle: electrons repel one another . So in every molecular structure the electron pairs on the central atom, whether shared in bonds or unshared ("lone pairs"), will distribute around the central atom so as to be as far apart as possible. All of the molecular shapes we observe can be rationalized from this simple premise.
It's important to keep in mind that it is the electron pairs on the central atom that are doing the repelling and generating the shape, not the electrons on the atoms attached to the center.
For most common molecules the possible geometries will fall into one of five major shape categories, based on the number of electron pairs on the central atom.
1) Draw the Lewis structure for the molecule.
This vital if you're going to get the answer right.
2) Count the number of "things" on the atom you're interested in.
Let's say that you're looking at methane, CH
4
. If you want to find the bond angles, shape, and hybridization for carbon, count the number of things that are stuck to it.
Now, the vague term "things" refers to atoms and lone pairs. IT DOES NOT REFER TO THE
NUMBER OF BONDS! When you look at methane, there are four atoms stuck to it.
People get confused with multiple bonds. Take carbon dioxide, for example. There are four bonds (carbon is double-bonded to each oxygen) but only two oxygen atoms bonded to carbon. In this case, we count two things stuck to carbon, because we only count the atoms,
NOT the number of bonds.
Likewise, with ammonia there are four things. Three of the things on nitrogen are hydrogen atoms and the fourth is a lone pair. For the purposes of VSEPR, lone pairs count exactly the same as atoms, because they consist of negative charge, too.
3) Count the number of lone pairs that are on the atom you're interested in.
IMPORTANT: This does NOT mean to count the number of lone pairs on all of the atoms in the molecule. Lone pairs on other atoms aren't important - what's important is only what's directly stuck to the atom you're interested in.
Lewis Structures for Atoms: Just show inner "kernel" where symbol stands for nucleus and all inner shell electrons:
Sodium: Na
.
Sodium ion: Na +
Phosphorus: Bromine:
=====================================================
Ionic Compound Lewis Structure Examples:
Bromide ion: We need the brackets to show that the bromide ion "owns" all of the electrons rather than sharing them. This is particularly important when we make an ionic compound such as sodium chloride.
Sodium chloride: NaCl Potassium bromide: KBr Aluminum chloride: AlCl
3
====================================================
Covalent Compound Lewis Structure Examples:
Water H
2
O Ammonia NH
3
Ammonium ion NH
4
+
CH
4
Methane H
2
S Hydrogen sulfide CO
2
Carbon dioxide
Carbon monoxide
Choosing a Bond Type - Lewis Structure Examples:
Barium iodide check electronegativity difference….formula?
ionic: pos cation goes first BaI
2
Carbon disulfide check electronegativity difference….formula?
covalent: less electronegative element goes first CS
2
Arsenic triiodide check electronegativity difference….formula? covalent: AsI
3
Hydrogen selenide check electronegativity difference….formula? covalent: H
2
Se
Oxygen difluoride check electronegativity difference….formula?
covalent: OF
2
=================================================
Multiple Bonds - Examples
Carbonate ion :
However, other equally symmetrical structures are possible, so: