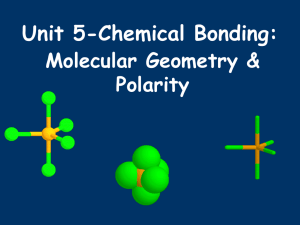
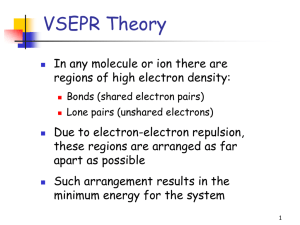
c - Rohan
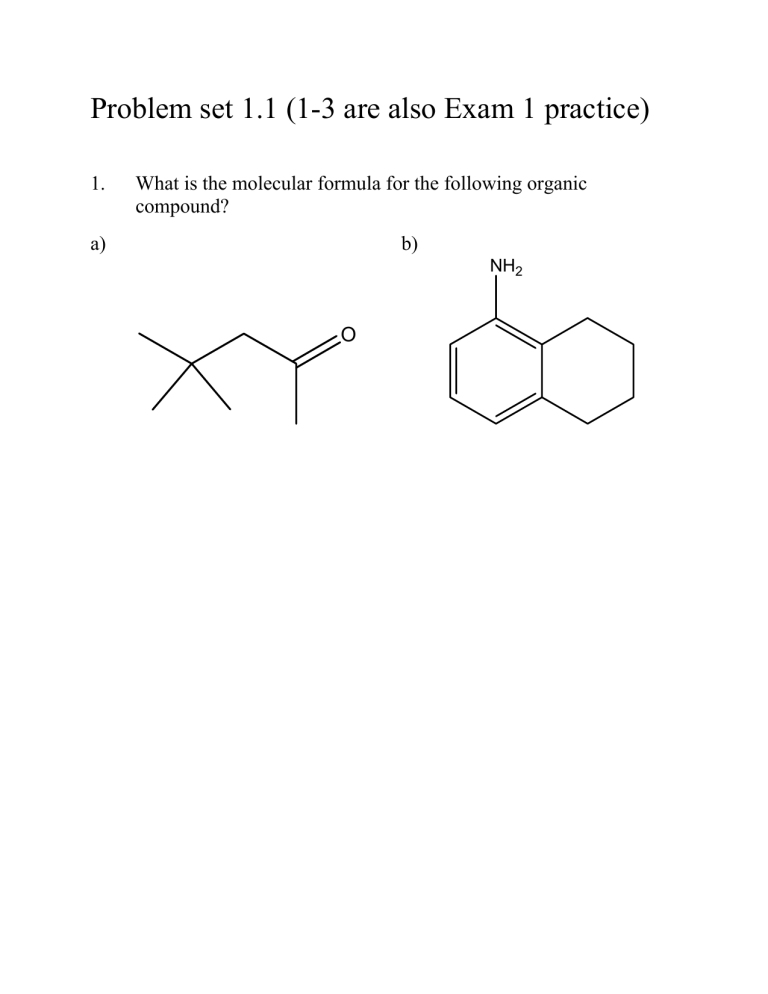
Problem set 1.1 (1-3 are also Exam 1 practice)
1. What is the molecular formula for the following organic compound? a) b)
NH
2
O
2. Consider an organic compound with molecular formula C
4
H
6
O and the following connectivity between non-H atoms:
C
C
O
C
C
How many lone pairs and pi-bonds will there be in a good Lewis structure for the compound? (Draw one good Lewis structure and count the number of pi bonds and lone pairs. All good Lewis structures will have the same number. Dashed lines are to show connectivity, not the number of bonds.)
( a ) 1 pi bond and 3 lone pairs ( b ) 0 pi bonds and 3 lone pairs
( c ) 1 pi bond and 2 lone pairs ( d ) 2 pi bonds and 2 lone pairs
( e ) 2 pi bonds and 3 lone pairs
3.
Which one of the following is a good 3D representation for the following organic compound?
H
H C
H
C C C
H
C
H
N
H
H
H a)
H
C
H
H
C c)
H
C
H
C
H e)
H
C
H
C
H
C
C
C C
H
H
C
H
N
H
C
H
H
N
H
b)
H
H
C
H
C
H d)
H
C
H
C
C C
H
C
C
H
H
C
N
H
H
C
H
N
H
H
H
C
C C
H
N
H
H
f) None of the a-e
4. Draw isomers of C
4
H
8
O. Draw as many as you can, but ignore cistrans isomers. For 5 isomers of your choice show 3D structures.




![Guidance on lone and out-of-hours working [DOCX 33.34KB]](http://s2.studylib.net/store/data/014979692_1-ee78a79daf404e4d826549abbd4d835e-300x300.png)