Chapter 8 - Iowa State University
advertisement

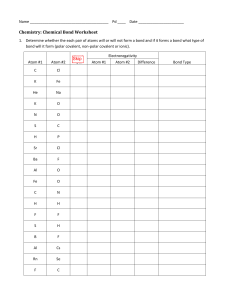
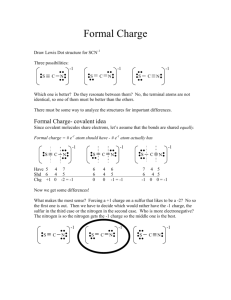
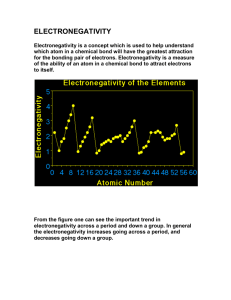
Chapter 8 Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Katie Chem 177 Kingston November 1, 2010 1. What is the octet rule? 2. Write the Lewis symbol for atoms of each of the following elements: a) Al b) Br c) Ar d) Sr 3. What is the Lewis symbol for each of the following atoms or ions: a) Ca b) P c) 𝑀𝑔2+ d) 𝑆 2− 4. a) What is meant by the term covalent bond? b) Give three examples of covalent bonding. 5. Which of these elements is unlikely to form covalent bonds: S, H, K, Ar, Si? Explain. 6. a) What is meant by the term electronegativity? b) Which element has the greatest electronegativity? The smallest? 7. Using only the periodic table select the most electronegative atom in each of the following sets: a) Se, Rb, O, In b) Ge, As, P, Sn 8. Which of the following bonds are polar? a) B-F b) Cl-Cl c) Se-O d) H-I Which is the more electronegative atom in each polar bond? Supplemental Instruction 1060 Hixson-Lied Student Success Center 294-6624 www.si.iastate.edu 9. The iodine monobromide molecule has a bond length of 2.49 Å and a dipole moment of 1.21 D. a) Which atom of the molecule is expected to have a negative charge? Explain. b) Calculate the effective charges on the I and Br atom in units of the electronic charge e.