Carbon: the Element of Life
advertisement
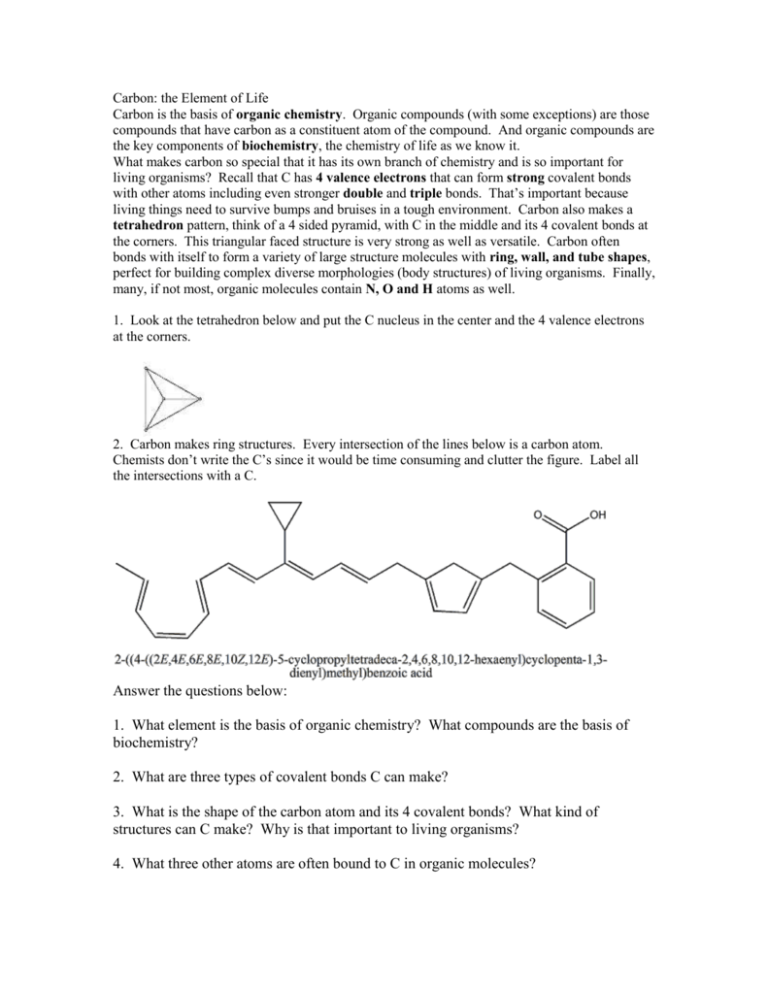
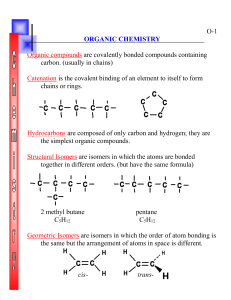
Carbon: the Element of Life Carbon is the basis of organic chemistry. Organic compounds (with some exceptions) are those compounds that have carbon as a constituent atom of the compound. And organic compounds are the key components of biochemistry, the chemistry of life as we know it. What makes carbon so special that it has its own branch of chemistry and is so important for living organisms? Recall that C has 4 valence electrons that can form strong covalent bonds with other atoms including even stronger double and triple bonds. That’s important because living things need to survive bumps and bruises in a tough environment. Carbon also makes a tetrahedron pattern, think of a 4 sided pyramid, with C in the middle and its 4 covalent bonds at the corners. This triangular faced structure is very strong as well as versatile. Carbon often bonds with itself to form a variety of large structure molecules with ring, wall, and tube shapes, perfect for building complex diverse morphologies (body structures) of living organisms. Finally, many, if not most, organic molecules contain N, O and H atoms as well. 1. Look at the tetrahedron below and put the C nucleus in the center and the 4 valence electrons at the corners. 2. Carbon makes ring structures. Every intersection of the lines below is a carbon atom. Chemists don’t write the C’s since it would be time consuming and clutter the figure. Label all the intersections with a C. Answer the questions below: 1. What element is the basis of organic chemistry? What compounds are the basis of biochemistry? 2. What are three types of covalent bonds C can make? 3. What is the shape of the carbon atom and its 4 covalent bonds? What kind of structures can C make? Why is that important to living organisms? 4. What three other atoms are often bound to C in organic molecules?