Organic Chemistry
advertisement

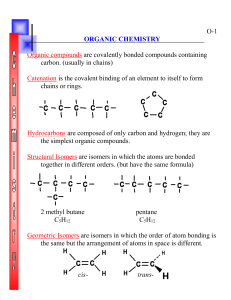
ORGANIC CHEMISTRY ORGANIC CHEMISTRY IS CHEMISTRY OF CARBON COMPOUNDS. Hydrocarbons are a category of organic compounds which contain carbon and hydrogen only. Some of these compounds contain only single bonds, shown as – (sharing 2 electrons), ending with ane., Others contain one or more double bonds, shown as = (sharing 4 electrons), ending with ene, Still others contain triple bonds, sharing 6 electrons, shown as =, ending with yne. There is also a category of hydrocarbons, which are aromatic, like benzene (C6H6). The rest of organic compounds have one or more functional groups, the groups, which have heteroatoms, any atom except carbon or hydrogen. The different categories of organic compounds are listed below: 1. Hydrocarbons: A. Alkanes, containing only single bonds: CH4 methane C2H6 or CH3-CH3 ethane C3H8 or CH3-CH2-CH3, propane B. Alkenes, containing one or more double bonds. C2H4 OR CH2=CH2, ethene or ethylene C. Alkynes, containing one or more triple bonds: C2H2 or CH=CH, ethyne or acetylene D) Substituents: Attaching different groups CH3- methyl C2H5- ethyl C3H7- propyl E) Aromatic hydrocarbons, like benzene, (C6H6) 2. Alcohols, containing –OH CH3OH, methyl alcohol or wood alcohol or methanol, a potent poison, Causing blindness and death. CH3-CH2 –OH or C2H5-OH, ethyl alcohol, beverage alcohol or ethanol, the alcohol in beer and wine. CH3-CH2-CH2-OH or C3H7-OH, n-propyl alcohol or 1-propanol. CH3-CH-CH3, isopropyl alcohol, or rubbing alcohol, or 2-propanol OH 3. Thiols containing –SH: CH3-SH, methanethiol, all organic sulfur compound smell very bad, such as Garlic, and skunk. O 4. Aldehyde: H-C-H, or formaldehyde O 5. Ketones: CH3-C-CH3, or acetone 6. Carboxylic acids such as CH3COOH or acetic acid, or vinegar. 7. Salts of carboxylic acid: From reactions of carboxylic acid and a base (NaOH or sodium hydroxide) such as: CH3-COOH + NaOH ---- CH3-COONa + HOH CH3-COONa is sodium acetate which is a salt. 8. Esters which are formed from the reaction of carboxylic acids and alcohols, like methyl acetate: CH3-COOCH3. The esters have fruit aroma. 9. Amines such as CH3-NH2 or methylamine. 10. Ethers: CH3-CH2-O-CH2-CH3, diethyl ether, an anesthetic. 11. Amino acids containing amino group (-NH2) and carboxyl group (–COOH) like glycine and alanine. NH2-CH2-COOH, glycine.