Understanding Bohr`s Theory- Spectra
advertisement

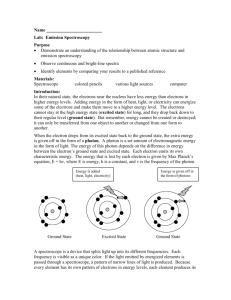
Pre-AP Chemistry Instructor: Mr. Malasky Name _____________________________________ Period _______ Due Date _____________ Laboratory Activity: Understanding Bohr’s Theory- Spectra Background When atoms in the ground state are heated to higher temperatures, some electrons may absorb enough energy to jump to a higher energy level. The atom is then said to be in an excited state. The excited configuration is unstable, and the electrons fall back to their normal positions of lower energy. As this occurs, energy that was absorbed is emitted in the form of visible energy, viewed as light. When this occurs for a specific element, the color of light and spectra from this emission serves as a “fingerprint” of the element to which the atoms belong. Bohr’s theory stated that no two elements have the same atomic emission spectra. The exciting of atoms generally occurs in one of two ways. When conducting electricity through and gas discharge tube or providing heat to an element (flame test), the results allow you to view the various distinct colors of the emission spectrum of a sample using a spectroscope. A flame test is generally more difficult because the production of light is of a short duration. Only metals, with their loosely held electrons, are excited in the flame of a burner. Therefore, flame tests are generally useful in identifying metallic content in a compound. A spectrum is formed when light passes from one medium into another, and the light is separated into its component wavelengths. Basic types of spectra include a continuous spectrum and a bright line (emission) spectrum. The continuous spectrum is the result of breaking down white light. A bright line spectrum appears when a substance is radiating a limited number of wavelengths and therefore producing a limited number of colored lines. The wavelengths are mathematically related to the definite quantity of energy produced as electrons move from one level to another. Spectroscopy is an important tool for the analytical chemist to identify chemical composition in products and unknown samples. Materials burner/striker spectroscope tongs electric power source gas spectrum tubes spectroscopes sunlight white light fluorescent light cupric chloride (aq) potassium chloride (aq) lithium chloride (aq) Methods- read all steps before starting. 1. Start at any of the locations set up for this lab. Move to all locations and complete #2. 2. For the four gas spectrum tubes, three ionic salt solutions, sunlight, white light, and fluorescent light, observe the light produced, then use the spectroscope to identify the component light wavelengths. Hold the spectroscope with the prism end toward you. Look through this eyepiece and point the slit end at the light source. Look inside the spectroscope and find a visible spectrum. Complete the information for each station and diagram each spectrum in the boxes provided. Be sure all color components are labeled in each diagram, drawing to scale as best as possible. cont’d-------- Observations/Conclusions. Complete the chart containing for each light source listed. (60 pts) station/light source #1 helium color observed type of spectra diagram #2 mercury #3 neon #4 nitrogen #5 lithium chloride #6 potassium chloride #7 cupric chloride #8 incandescent bulb (tungsten filament) #9 sunlight #10 fluorescent Questions. Briefly complete each of the following. (40 pts) 1. A rainbow that forms after a thunderstorm is considered what type of spectrum? 2. Why doesn’t the fluorescent light produce a continuous spectrum? 3. How could you confirm that what appears to be a “white” light source is truly white? 4. Why is a bright line spectrum considered a “fingerprint” for a chemical element? 5. What is the key component of a spectroscope? What does it do to the light? 6. What phase of matter is represented in the neon tube used in this lab? 7. Which salt solution produced the visible flame with the highest wave frequency? 8. What color of visible flame would you expect to view from a solution of lithium nitrate?