Atomic Emission Spectra Lab
advertisement

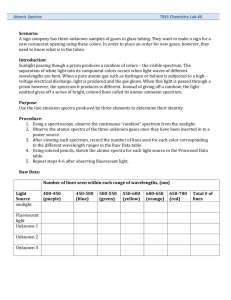
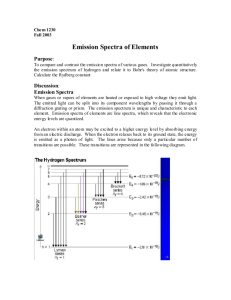
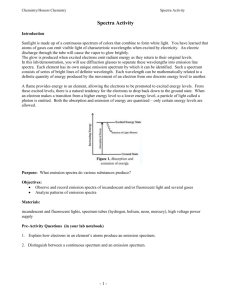
LaVigne Atomic Emission Spectra Lab Conclusion Questions 1. What is an atomic emission spectrum and what causes it? 2. In art class you learn that if you mix blue and yellow it makes green. How does that idea apply to each colored flame you viewed in the lab? (i.e How is the atomic emission spectrum of an element related to these flame tests?) 3. What is the identity of the unknown crystals? Explain how you identified the unknowns. 4. Why are there black spaces between the colors in each element’s spectrum? 5. Did any of the elements have the same spectra? Why/Why not? 6. In terms of electrons, what causes the atomic emission spectra of each element? Explain as detailed as possible. 7. In the continuous spectrum, which color has the highest wavelength? Lowest wavelength? 8. In the continuous spectrum, which color has the highest frequency? Lowest frequency? 9. Which of the colors has the most energy? 10. Write a general statement that relates how frequency, wavelength, and energy are related for all types of radiation on the electromagnetic spectrum. 11. If you tested three elements via a flame test and 2 of three had the same color flame, what might you conclude? 12. If you then used a prism to break down the spectra of the 2 elements that had the same flame color, and found that the specific frequencies emitted did not match, what would your conclusion be? 13. The following are some simple emission spectra: Look at the above spectra. Look at the one below, what element’s spectrum is it? How do you know? 14. What does each line on the above atomic emission spectrum represent? 15. How many photons does each of the following emit when the element is in an excited state: helium, neon, and mercury (see above spectra)