SOLUBILITIES: AN INVESTIGATION
advertisement

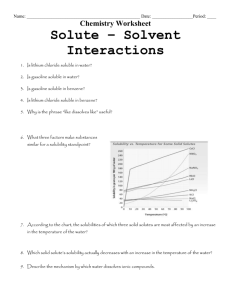
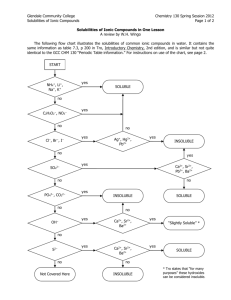
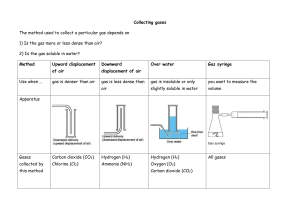
GISAT 112 SOLUBILITIES: AN INVESTIGATION (adapted from Laboratory Manual, Chemistry in Context, 2nd Edition, 1997.) Introduction This laboratory exercise is a departure from the usual experiment. It is an attempt to simulate the kind of problem solving that takes place in a scientific laboratory. There are no instructions, procedures, or data sheets. There is simply a problem to solve, which requires reasoning skills and the application of knowledge already acquired. A team will consist of two students. You and your laboratory partner must come up with a solution to the problem and be prepared to compare your method and results to those of other teams in the class. The Problem Determine the relative solubilities of the following compounds in water at room temperature: calcium sulfate (CaSO4), potassium aluminum sulfate (KAl(SO4)2), potassium nitrate (KNO3), ammonium nitrate (NH4NO3), and sodium chloride (NaCl). Express their solubilities in ranked order from most soluble to least soluble. All of these compounds are likely to be found in drinking water supplies since they occur naturally in rocks and soil or are used as fertilizers. In the interest of cost and convenience, you are provided with approximately 2 grams of each compound. (You will not be supplied with any more so think carefully about how you will use it!) Once you’ve decided on a procedure, record it and any observations you make while trying to solve this problem. Your grade will depend on three things: 1. A defensible answer to the problem 2. The quality of the procedure you develop 3. The care with which you carry out the investigation Materials and Equipment Each lab table will have an analytical balance. In addition, a variety of materials and equipment will be available upon request. The lab assistants and instructors are willing to bring you graduated cylinders, beakers, burets, glass stir rods, spatulas, test tubes, eyedroppers, and more. If you have questions about equipment, don’t hesitate to ask. Reporting Your Results On a single sheet of paper, record your ranking of the salts from most soluble to least soluble. Carefully describe your procedure and any observations you’ve made while carrying it out. If time allows, your instructor will facilitate a discussion of the activity.