Acrobat PDF
advertisement

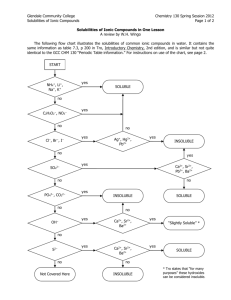
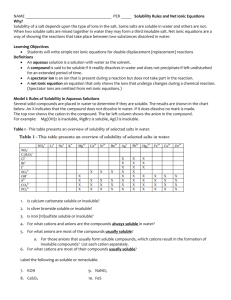
AQUEOUS RXNS & SOL'N STOICHIOMETRY Solutions in which water is the dissolving medium (solvent) are called aqueous solutions. Properties of Aqueous Solutions Electrolytic Properties Ionic Compounds in Water Molecular Substances in Water Strong and Weak Electrolytes Precipitation Reactions occur when the mixed solutions contain a combination of ions which form a sparingly soluble (or insoluble) compound. Solubility Guidelines for Ionic Compounds Predicting Precipitation Reactions when two ionic compounds are mixed in aqueous solution is done by checking the solubilities of the compounds formed when the ions “switch partners”: ♦ if either of the new compounds is insoluble (or slightly soluble) precipitation occurs. ♦ if both new compounds are insoluble, two precipitation reactions occurs ♦ if both new compounds are soluble, no precipitation occurs. Solubilities of Ionic Compounds in Water + + NH 4 , Na , K All ammonium, sodium, and potassium salts are soluble. + Nitrates: − All nitrates are soluble. NO 3 Fluorides: Most fluorides are soluble; exceptions include SrF2, BaF2, PbF2, MgF, CaF2. − F Chlorides: − All chlorides are soluble except AgCl, Hg2Cl2, and PbCl 2 Cl Bromides: Most bromides are soluble; exceptions include PbBr2, HgBr2, AgBr, Hg2Br2. − Br Iodides: − Most iodides are soluble; exceptions are AgI, Hg2I 2, PbI2, HgI 2. I Sulfates: 2− Most sulfates are soluble; exceptions include SrSO4, BaSO4, and PbSO4. SO4 Chlorates: − All chlorates are soluble. ClO 3 Perchlorates: − All perchlorates are soluble, except KClO4, which is slightly soluble. ClO 4 Acetates: CH 3COO Phosphates: All acetates are soluble except for Be(CH3COO)2 3− All phosphates are insoluble except for those of Group I elements and + NH4 . 2− All carbonates are insoluble except for those of Group I elements and + NH4 . PO 4 Carbonates: CO 3 Hydroxides: OH Sulfides: − − + All hydroxides are insoluble except for those of NH4 , Group I, Sr(OH)2, and Ba(OH)2; Ca(OH)2 is slightly soluble. + All sulfides are insoluble except for those of NH4 , Groups I & II. 2− S Sulfites: + SO3 2− All sulfites are insoluble except for those of NH4 and Group I. Exchange Reactions Ex. 1 Predict whether or not a precipitate will form when the following two solutions are mixed: (a) AgNO 3(aq) + NaCl(aq) → (b) Pb(NO3)2(aq) + KI(aq) → (c) Ba(ClO 3)2(aq) + Li2SO 4(aq) → (d) BaCl 2(aq) + NaOH(aq) → Ionic Equations Ex. 2 An aqueous solution of sodium carbonate is mixed with an aqueous solution of calcium chloride. A white precipitate immediately forms. Write a net ionic equation to account for this. What are the spectator ions? Acid and Base Reactions Acids Strong acids are HNO3 , H 2 SO4 , HClO3 , HClO4 , HCl, HBr, HI Weak acids include HF, CH 3 COOH, HCOOH, H 2 C2 O4 , H 3 PO4 Bases Strong bases — include Ba(OH)2 and hydroxides of the alkali metals (NaOH, KOH, etc.), the soluble ionic hydroxides. Other hydroxides are either slightly soluble – or insoluble and are weak bases because the OH ions are mostly tied up in the solid. Acid-Base Reactions Reactions of acids ♦ acid + carbonate (or hydrogen carbonate) ♦ acid + metal oxide ♦ acid + metal Reactions of bases ♦ base + ammonium salt ♦ base + non-metal oxide Oxidation-Reduction Reactions Redox reactions are characterized by a transfer of electrons. An atom is oxidized (loses electrons) if its oxidation number increases (becomes more positive) in a chemical reaction; an atom is reduced (gains electrons) if its oxidation number decreases. Oxidation Numbers —can be determined for the atoms in a compound by following a simple set of rules: ♦ Oxidation numbers of atoms in neutral molecule add up to zero, and those in an ion add up to charge on that ion. ♦ Group I atoms +1; group II elements +2; group III atoms usually +3. ♦ Fluorine always −1 in compounds. Other halogens −1, except in compounds with oxygen or other halogens. ♦ Hydrogen is +1 except in metal hydrides (LiH) rule 2 takes precedence here. ♦ Oxygen is −2 in compounds. Exceptions: compounds with fluorine (see rule 3) and compounds with O-O bonds (rules 2 and 4). Ex 3 Assign oxidation numbers to the atoms in the following: − (a) NaCl (b) ClO (c) Fe2(SO 4)3 (e) I2 (f) KMnO4 (g) CaH2 (d) SO 2 Activity Series Ex 4 Which of the following metals will be oxidized by Pb(NO 3)2: Zn, Cu, Fe? Concentrations of Solutions Molarity ( M ) Ex 5 Calculate the molarity of a solution prepared by dissolving 10.0 g of AgNO3 in enough water to make 250.0 mL of solution. Ex 6 A flask contains 625 mL of 3.05 M calcium nitrate solution. What volume of 15.8 M Ca(NO3)2 contains the same number of moles of Ca(NO3)2 as this solution? Ex 7 What is the molar concentration of nitrate ions in example 6 above? Dilution Ex 8 How many milliliters of 4.5 M HCl are required to prepare 200 mL of 0.75 M HCl? Ex 9 (a) Describe how to prepare 0.500 L of a 0.0250 M aqueous solution of potassium dichromate (b) Describe how to dilute this solution to obtain a solution with a final concentration of 0.0140 M K2Cr2O7. Ex 10 When the orange salt potassium dichromate is added to a solution of concentrated hydrochloric acid, it reacts according to the net ionic equation: K2Cr2O7(s) + 14 HCl(aq) → + 3+ − 2 K (aq) + 2 Cr (aq) + 8 Cl (aq) + 7 H2O(l) + 3 Cl2(g) Suppose that 6.20 g of K2Cr2O7 reacts completely in a solution with a total volume of 3+ 100.0 mL. Calculate the final concentration of Cr (aq) ion that results and the number of moles of chlorine gas produced. Titrations Ex 11 What is the molarity of a solution of sodium hydroxide if it requires 23.97 mL of that solution to reach the phenolphthalein end-point when adding it to a solution containing 0.5333 g of KHC8H4O4? Ex 12 The indicator methyl red turns from yellow to red when the solution in which it is dissolved changes from basic to acidic. A 25.00-mL volume of a sodium hydroxide solution is titrated with 0.8367 M HCl. It takes 22.48 mL of this acid to reach a methyl-red end-point. Find the molarity of the sodium hydroxide solution.