Naming Molecular Compounds and Acids Notes
advertisement

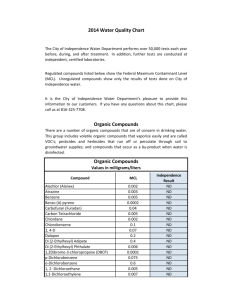
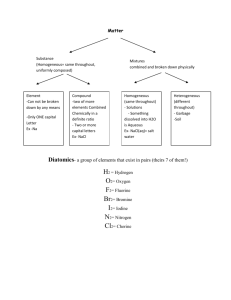
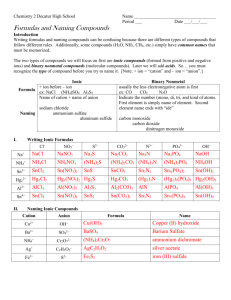
Chapter 5: Molecules and Compounds Date:____________ Section 4: Naming Molecular Compounds and Acids - Notes Objectives: Name molecular compounds. Name binary acids. Name oxyacids containing an oxyanion ending in -ate. Name oxyacids containing an oxyanion ending in -ite. Naming Molecular Compounds: Remember, nearly all molecular compounds form from two or more ______________________. Binary (two-element) molecular compound names have the following form: + When writing the name of a molecular compound the first element is the more __________________________ one The prefixes given to each element indicate the number of specific atoms present o mono o hexa o di o hepta o tri o octa o tetra o nona o penta o deca If there is only _________ atom of the first element, the prefix mono- is normally omitted. o CO2 The full name is ________________________________. When the prefix ends with a vowel and the base name starts with a vowel, the _____________ ___________________ is sometimes dropped. o Vowel is dropped in _______________________ but not in dioxide or triiodide. o N2O The entire name is ________________________________________________. Practice: Name the following compounds: o CCl4 o SF6 o BCl3 o N2O4 Practice: Write the formulas for each compound: o disulfur tetrafluoride o phosphorus pentafluoride o xenon hexafluoride Naming Acids: The first step in naming an acid compound is identifying it as _______________________. Acids are molecular compounds that form ___________ ions when dissolved in water. o Acids will always be _________________________ (______) They are composed of _______________________________, usually written first in their formula, and one or more ____________________________________, written second. We can categorize acids into two groups: o ___________________________: those containing only hydrogen and a nonmetal o ___________________________: those containing hydrogen, a nonmetal, and oxygen Binary acids are composed of hydrogen and a nonmetal. The names for binary acids have the following form: + HCl(aq) HCl(g) o HBr(aq) refers to __________________________________________ molecules in the gas phase, and not to the acid. Practice: Name the following compounds: o o HF(aq) o hydrofluoric acid Practice: Write the formula of each compound: o H2S(aq) hydroiodic acid Oxyacids are acids that contain oxyanions, which can be found in the table of polyatomic ions. o These acids are a combination of one or more H+ ions with _______________________. o The number of H+ ions depends on the charge of the oxyanion, so that the formula is always ____________________________________. The names of acids containing oxyanions ending with -ite take this form: + o H2SO3(aq) contains the __________________ (_________) ion The names of acids containing oxyanions ending with -ate take this form: + o Practice: Name the following compounds: o HNO3(aq) contains the __________________ (_________) ion HC2H3O2(aq) o HNO2(aq) Practice: Write the formula for each compound: o Nitric acid o Hypochlorous acid o Nitrous acid o Perbromic acid Practice: Name the following compounds: CO(g) HF(aq) HClO4(aq) CaF2(aq) Fe(NO3)3(s) H2SO3(aq)