SNC2P1 Chemistry Test #1 Review ans key
advertisement

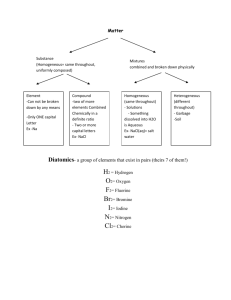
SNC2P1 Name: ________________________________________ CHEMISTRY TEST #1 REVIEW PART A – Match the following terms to their definition. 1. Physical Change 4 2 or more elements bonded together 2. Chemical Change 5 an atom that has lost electrons 3. Element 8 a compound with a metal and a nonmetal 4. Compound 7 the last electron shell of an atom 5. Positive ion 3 cannot be broken down any further 6. Negative ion 13 valence electrons 7. Valence shell 10 Group 17 8. Ionic compound 1 no new substances are produced 9. Molecular compound 9 a compound with 2 nonmetals 10. Halogens 2 new substances with different properties 11. Noble gases are produced 12. Alkali metals 12 Group 1 13. elements in the same group 6 an atom that has gained electrons have the same number of ? 11 Group 18 PART B – Ionic or Molecular? Identify the types of atoms in each compound as either metal or non-metal. Then decide if the compound is ionic or molecular. Chemical Formula Metal + Non-metal Ionic or Molecular Compound? or 2 Non-metals? SrCl2 Metal + non-metal Ionic compound P2O5 2 non-metals Molecular compound SCl2 2 non-metals Molecular compound CBr4 2 non-metals Molecular compound Rb2S Metal + non-metal Ionic compound PART C – Use the WORD BANK below to fill in the blanks. 1. A subscript tells you how many atoms of the element are in the compound. 2. Metals are on the left side of the staircase on the periodic table. 3. The protons and neutrons are in the nucleus. 4. The electrons are surrounding the nucleus in shells or orbits. 5. Metals will lose electrons to become stable and nonmetals will gain electrons to become stable. 6. Nonmetals will have a negative charge when they become ions and metals will have a positive charge. 7. To become stable, all atoms want a full outer shell. 8. A group of element symbols and subscripts that represent the MAKEUP of a chemical compound is called a chemical formula. chemical formula negative gain Word Bank full left lose positive subscript nucleus electrons neutrons right PART D – Circle the best answer for each. 1. Which of the following is an element? a) water b) salt c) mercury d) pizza 2. Which is a chemical change? a) Ripping a piece of paper in two. b) Baking chocolate cupcakes. c) Dissolving sugar into coffee. d) A popsicle melting on a hot day. 3. Which of the following is a compound? a) aluminum b) carbon dioxide c) copper d) Kool-Aid in water 4. Which element has 34 electrons, 34 protons and 45 neutrons? a) rhodium b) bromine c) sodium d) selenium 5. How many valence electrons does nitrogen have? a) 2 b) 3 c) 4 d) 5 6. Which ion will calcium form to become stable? a) +2 b) +4 c) -2 d) -4 7. Which family does Chlorine belong to? a) alkali metal b) alkaline earth metal c) halogen d) noble gas 8. How many protons, neutrons and electrons does carbon have? a) 6, 7, 6 b) 8, 7, 8 c) 8, 6, 8 d) 6, 6, 6 PART E- Indicate if the following compounds are Ionic or Molecular, by putting a checkmark in the correct column. Compound sodium chloride carbon tetrachloride silver nitride magnesium oxide nitrogen monoxide Ionic Molecular PART F – Naming Compounds (Only molecular compounds have the prefixes mono, di, tri, etc.) 1. Mg3N2 magnesium nitride 5. CO carbon monoxide 2. LiCl lithium chloride 6. N2O2 dinitrogen dioxide 3. BaO barium oxide 7. CCl4 carbon tetrachloride 4. Al2S3 aluminum sulfide 8. NH3 nitrogen trihydride PART G – Write the formula for the following compounds. -crossover the charges (not the sign) and reduce the numbers for ionic compounds -write the number that matches the prefix for molecular compounds. 1. lithium oxide Li2O 5. sodium chloride NaCl 2. carbon dioxide CO2 6. carbon tetrafluoride CCl4 3. potassium bromide KBr 7. diphosphorus trisulfide P2S3 4. silicon tetraiodide SiI4 8. barium iodide BaI2 PART H – More Formula Writing Identify the following compounds as ionic or molecular. Then write their formula. Compound Name Ionic or Molecular Compound Formula carbon monoxide Molecular CO zinc oxide Ionic ZnO potassium fluoride Ionic KF dinitrogen pentoxide Molecular N2O5 aluminum sulfide Ionic Al2S3 phosphorus trichloride Molecular PCl3 disulfur dinitride Molecular S2N2 magnesium phosphide Ionic Mg3P2 lithium iodide Ionic LiI diphosphorus hexoxide Molecular P2O6 PART I – Reading Chemical Formulas Name of Compound sodium fluoride Chemical Formula NaF calcium nitride Ca3N2 diphosphorus trioxide P2O3 boron triiodide BI3 beryllium chloride BeCl2 Number of Atoms of Each Element 1 atom of sodium 1 atom of fluorine 3 atoms of calcium 2 atoms of nitrogen 2 atoms of phosphorus 3 atoms of oxygen 1 atom of boron 3 atoms of iodine 1 atom of beryllium 2 atoms of chlorine