Compounds
advertisement

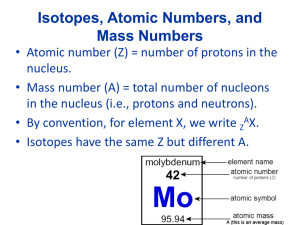
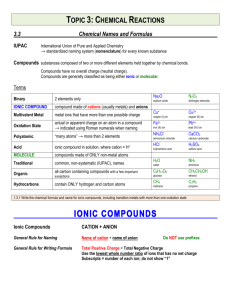
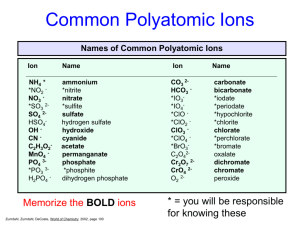
Compound Names and Formulas • • • • • Binary Ionic Compounds Binary Molecular Compounds Polyatomic Ionic Compounds Organic Hydrocarbons Acids Binary Ionic Compounds • Compounds composed of a metal cation and a nonmetal anion • First name is the name of the metal • Second name is the name of the nonmetal with an ide suffix • Ex. NaCl is sodium chloride Molecular Binary compounds • Composed of two elements both of which are nonmetals. • No ions present • First name is name of the first element with prefix • Second name is name of second element with prefix • Prefix indicates subscript number: mono, di, tri, tetra, penta, hexa, hepta, octa, nona and deca • Ex: Carbon monoxide – CO2 Polyatomic Ionic Compounds • Composed of metal cation and anion made up of two or more nonmetal atoms with a net negative charge • First name is name of metal cation • Second name is name of polyatomic anion • Ex. Sodium sulfate – Na2SO4 Organic Compounds • Hydrocarbons are made up of carbon and hydrogen atoms • Alkanes have the formula: CnH2n+2 • Prefix defines the n value: n=1-9 meth, eth, pro, but, pent, hex, hept, oct, non, and dec • Name ends with ane suffix • Ex: methane – CH4 Binary Acids • All acids contain hydrogen ion: H+ • In binary acids the anions is a monoatomic nonmetal anion • Generic formula: HA • Prefix: hydro Suffix: ic • Ex. Hydrochloric acid - HCl Polyatomic Acids • All acids contain hydrogen ion: H+ • In polyatomic acids the anion is a polyatomic anion • Generic formula: HA • Prefix: none(unless polyatomic anion name has one) Suffix: ic(ate) or ous(ite) • Ex. chloric acid – HClO3 • Ex: hypochlorous acid – HClO Writing formula for ionic compounds from formula • MxAy will be MyAx • Example: Calcium(2+) nitrate(1-) – Ca(NO3)2 • Ammonium(1+) carbonate (2-) – (NH4)2CO3 Writing Molecular formula from name for binary molecular formulas • Prefix indicates subscripts for that element • Dinitrogen pentoxide: N2O5 Writing Alkane formula from name • Prefix indicates carbon subscript, H subscript is 2n+2 • Ethane: C2H6 Writing Acid Formula from Name • First determine if it is binary, it will have a hydro prefix and ic suffix • All acid formulas begin with hydrogen ion • Treat them like an ionic binary compound • Determine anion element and charge • Ex: hydrosulfuric acid – H2S Polyatomic acids • • • • Determine the anion and its charge Treat it like a polyatomic ionic compound Ex: nitric acid – HNO3 Ex: phosphoric acid – H3PO4 Quiz Time! Identify the formula for: barium nitrate • • • • • A. Ba3N2 B. Ba(NO2)2 C. BaNO2 D. BaNO3 E. Ba(NO3)2 Identify the formula for: chromium (III) phosphate • • • • • A. Cr3PO3 B. Cr3(PO4)2 C. Cr2(PO4)3 D. CrPO4 E. Cr3(PO4)3 Identify the formula for: Ammonium nitrate • • • • • A. NH4NO3 B. NH4NO2 C. NH3NO3 D. NH3NO2 E. (NH4)NO2 Identify the formula for: Copper (II) bromide • • • • • A. CuBr B. CuBrO3 C. Cu(BrO3)2 D. CuBr2 E. Cu(BrO4)2 Identify the formula for: Copper (II) bromide • • • • • A. CuBr B. CuBrO3 C. Cu(BrO3)2 D. CuBr2 E. Cu(BrO4)2 Identify the formula for: Sulfur Hexafluoride • • • • • A. SF B. SF6 C. SF4 D. S4F6 E. SF8 Identify the formula for: Octane • • • • • A. C6H6 B. C6H12 C. C8H8 D. C8H16 E. C8H18 Identify the formula for: Hydroiodic Acid • • • • • A. HI B. H2I C. HIO3 D. HIO2 E. HIO4 Identify the formula for: Sulfuric Acid • • • • • A. HS B. H2S C. H2SO4 D. H2SO3 E. H3SO3 Identify the formula for: Tetraphosphorus Decoxide • • • • • A. PO5 B. P2O5 C. P4O8 D. P4O10 E. P5O10