Analgesic
advertisement
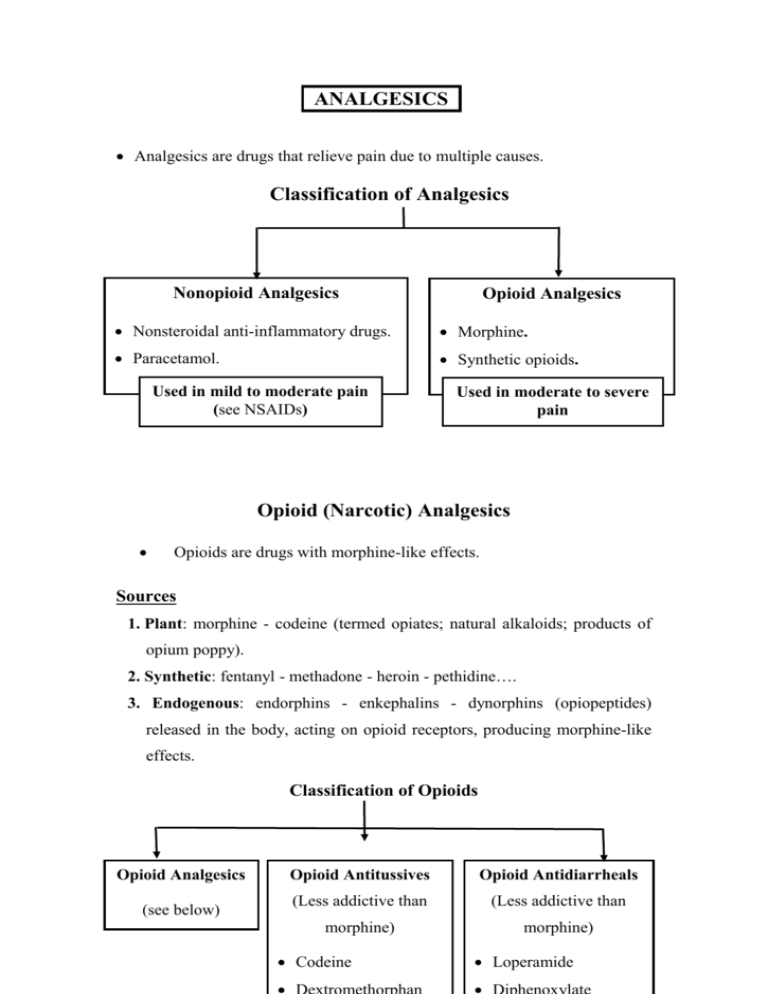
ANALGESICS Analgesics are drugs that relieve pain due to multiple causes. Classification of Analgesics Nonopioid Analgesics Opioid Analgesics Nonsteroidal anti-inflammatory drugs. Morphine. Paracetamol. Synthetic opioids. Used in mild to moderate pain (see NSAIDs) Used in moderate to severe pain Opioid (Narcotic) Analgesics Opioids are drugs with morphine-like effects. Sources 1. Plant: morphine - codeine (termed opiates; natural alkaloids; products of opium poppy). 2. Synthetic: fentanyl - methadone - heroin - pethidine…. 3. Endogenous: endorphins - enkephalins - dynorphins (opiopeptides) released in the body, acting on opioid receptors, producing morphine-like effects. Classification of Opioids Opioid Analgesics (see below) Opioid Antitussives Opioid Antidiarrheals (Less addictive than (Less addictive than morphine) morphine) Codeine Loperamide Mechanism of Action 1. Opioid receptors, mu (mediate most effects of opioids), kappa & delta, are Gi protein-coupled receptors present in the CNS and periphery (e.g. GIT). 2. Opioids (directly or through release of opiopeptides) activate receptors in: Afferent pain-conducting fibers peripheral analgesia. Spinal cord spinal analgesia. Brain stem1, thalamus & cerebral cortex supraspinal analgesia. Limbic system euphoria & emotional response to pain (patient may still feel the pain but the feeling is not unpleasant). 3. Activation of receptors neuronal inhibition through: a. Inhibition of Ca2+ entry release of excitatory neurotransmitters including substance P. b. Stimulation of K+ outflux hyperpolarization of neuronal membrane. 1 Activation of opioid receptors in midbrain activation of inhibitory descending pathways to raphe nuclei in medulla to dorsal horn of spinal cord→ transmission in pain pathways ascending from spinal cord to thalamus, limbic & somatosensory cortex. Actions, Uses, Adverse Effects & Contraindications (CI) of Morphine Uses Actions 1. Analgesic in: Acute trauma. Chronic visceral pain Postoperative pain Cancer pain Myocardial infarction I. Main Effects A. Analgesia Pain perception & emotional response to pain 2. In anesthesia Preanaesthesia CV surgery C. Euphoria(or dysphoria) B. Sedation Adverse Effects & CI Masks pain CI: acute undiagnosed abdomen Sedation - Narcosis Drug dependence II. Inhibitory Effects 3. Acute pulmonary edema in LVF: ↓preload & after load Respiratory distress A. VMC → venular & arterial VD Hypotension B. Respiratory center Respiratory depression & asphyxia neonatorum depression→ CO2 → Cerebral VD & increased intracranial tension 4. Antitussive Replaced by Codeine Dextromethorphan (less addictive) C. Cough center D. Uterine tone delayed Delayed labor labor III. Stimulatory Effects A. Oculomotor nucleus miosis B. CTZ vomiting 5. Antidiarrheal Loperamide Diphenoxylate (less addictive, more widely used) Intracranial tension CI: head injury C. Urinary & GI Tracts tone of wall & sphincters (spasmogenic) but peristalsis stools stagnate & harden due to fluid absorption. D. Histamine release Miosis Nausea - vomiting Urine Retention (CI: enlarged prostate Biliary colic(CI Constipation Hypotension – itching bronchospasm(CI: asthma) Other CI: extremes of age - hypothyroidism - liver dysfunction ( opioid metabolism) Tolerance develops to all effects except constipation and miosis. Classification of Opioid Analgesics 1. Strong A. Pure Agonists 2. Moderate 3. Weak Codeine Propoxyphene (Oral) (Oral) Morphine Analgesic plus Analgesic plus Methadone paracetamol or paracetamol or Pethidine aspirin in aspirin in mild Heroin moderate pain. to moderate Fentanyl(& subgroup) B. Partial agonists Antitussive. pain. Buprenorphine I. Pure Agonists 1. Morphine (see table). Given IV - IM - SC - epidurally - orally (extensive 1st pass metabolism). 2. Pethidine [IM - Oral] Used in acute moderate & severe pain e.g, trauma, postoperative pain, biliary colic or labor pain. Pethidine differs from morphine in: 1. Less biliary colic- less constipation- less urinary retention (shorter acting). 2. Less respiratory depressant in neonates2 & does not delay labor preferred during labor ( risk of asphyxia neonatorum). 3. Atropine-like action: dry mouth, blurred vision, …..... 4. Risk of convulsions (with high dose or in renal failure due to accumulation of the pethidine metabolite, norpethidine). 2 Morphine metabolism (conjugation ) is deficient in newborns longer depressant effect on respiration. 3. Methadone [Oral] Uses 1. Treatment of opioid addicts (detoxification & maintenance): Orally-active & long acting, thus, it is used to replace morphine or heroin in addicts. Gradual withdrawal of methadone is associated with less severe & smoother withdrawal symptoms. 2. Analgesic in severe chronic pain (efficacy equal to morphine). 4. Fentanyl More potent than morphine with rapid onset & shorter action (preferred in anesthesia). High anesthetic doses→ chest wall rigidity↓ thoracic compliance ventillation. Uses (IV– epidural- spinal - transdermal patch – pt. controlled infusion) 1. Analgesic in severe pain e.g. perioperative, labor & cancer pain. 2. In anesthesia (for its analgesic & sedative effects): Preanesthetic medication. IV anesthetic in cardiovascular surgery (safer). Conscious sedation - neuroleptanalgesia – neuroleptanesthesia. Conscious Sedation & Neuroleptanalgesia (Amnesia, sedation & analgesia without complete loss of consciousness) Uses: minor procedures or for diagnostic purposes (e.g. endoscopy). Conscious Sedation IV benzodiazepine (e.g. midazolam) - opioid analgesic (e.g. fentanyl). Easily reversed by flumazenil & naloxone (advantage). Neuroleptanalgesia Neuroleptic (e.g. droperidol) plus opioid analgesic (e.g. fentanyl). Converted to neuroleptanesthesia by adding 65% nitrous oxide in O2. Fentanyl subgroup Sufentanil is more potent than fentanyl. Remifentanil (IV infusion): ultrashort acting as it is metabolized by blood & tissue esterases →less ventillatory depression. 5. Heroin Diacetylmorphine converted to morphine in CNS. Rapid onset (greater lipid solubility crosses BBB more than morphine) & short duration risk of abuse (not used clinically in most countries). Tramadol Analgesic acting by inhibiting uptake of 5- HT and NA. Weak Mu agonist (only partially antagonized by naloxone). Less constipation, respiratory depression & addiction than morphine. ↑ Risk of convulsions. Uses (oral, IM, IV) Analgesic in postoperative & chronic moderate pain - neuropathic pain. II. Partial Agonists Buprenorphine (partial Mu receptor agonist) Advantages over Pure Agonists 1. Less addiction (less euphoria less craving). 2. Respiratory depression is not by dose (ceiling due to antagonist effect) but if it occurs, it is not easily reversed as it binds with affinity to receptors. Uses (parentral, sublingual) 1. Analgesic in severe pain. 2. Treatment of opioid addicts (long-acting, slowly dissociates from receptors) as an alternative to methadone (sublingual). Drug Interactions of Opioids 1. Opioids + other CNS depressants (sedatives, alcohol, antidepressants & antipsychotics) additive CNS depression. 2. Pethidine + MAOIs hyperpyrexia- respiratory depression - convulsions3. 3. Partial agonist + pure agonist withdrawal syndrome & analgesic effect. Acute Morphine Toxicity Coma. Respiratory depression. Pin pointed pupil (diagnostic). Treatment Support respiration. Naloxone (IV): opioid antagonist, repeated when necessary. 3 Inhibition of pethidine metabolism by MAOI norpethidine formation by another metabolic pathway. Pure Opioid Antagonists Naloxone Naltrexone IV & short-acting Oral & long-acting Management of acute toxicity Maintenance therapy in addicts 1. Acute opioid toxicity: 1. Opioid abuse Repeated as necessary to avoid Blocks euphoria of opioids relapse into coma since duration → loss of desire to take of action is shorter than opioids. drug (prevents relapse). 2. Asphyxia neonatorum 2. Alcohol abuse Respiratory stimulant in opioid- ↓ Craving in chronic induced respiratory depression alcoholics. in newborns. N.B.: Addicts should be closely monitored during reversal of acute opioid toxicity with naloxone to avoid precipitation of withdrawal symptoms. Naltrexone should be given to opioid addicts after full detoxification, otherwise it would precipitate a withdrawal syndrome