011 - Diatomics & Balancing Equations
advertisement

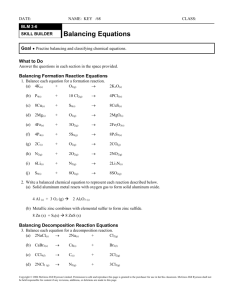
Diatomics In nature, some atoms don’t exist as individuals. They may “latch onto” one or more other particles of the same kind. The ones that bond with only one more are called the diatomic elements, or diatomics. ex. A fluorine atom will pair with a second fluorine atom to make an F2 molecule. Draw the shell diagram for this diatomic in the box on the right. To remember which elements are diatomic, think of Count “HOFBrINCl”. ex. Draw the shell diagrams for O2 and N2 below. Substance Representative Particle Examples elements atoms K, Li, Al, Ne, etc. diatomic elements molecules H2, O2, F2, Br2, I2, N2, Cl2 ionic compounds formula units NaCl, BaO, Al(OH)3, etc. molecular compounds molecule H2O, SF3, Br2Cl3 The Law of Conservation of Mass – Balancing Equations def’ns: In a chemical reaction, the substance(s) that you start with are called reactant(s). They are located on the left side of the equation. The substance(s) that you end up with are called the product(s). They are located on the right side of the equation. ex. When sodium is added to oxygen, we get sodium oxide. Na(s) + O2(g) Na2O(s) reactants product The Law of Conservation of Mass states that in a chemical reaction, matter is neither created nor destroyed. Thus, in a chemical equation, there must be the same amount of individual atoms on both sides. The process used to make sure that this is the case is called balancing equations. exs. Balance the following chemical equations. (1) Na + O2 Na2O (2) K + Cl2 KCl (3) Al + Br2 AlBr3 Assignment – Balancing Equations #1 Directions: Balance the following equations. Show any and all work below the equation. The last one is an example of a type of question you would see in a grade 11 chemistry class. (1) Li + S Li2S (2) Mg + N2 Mg3N2 (3) Na + H2O NaOH (4) Al2O3 (5) P4 + O2 P4O10 (6) FeS2 + O2 Fe2O3 (7) Al + + H2 + SO2 O2 barium chloride + magnesium sulfate barium sulfate + magnesium chloride