Double Replacement Reactions:
advertisement

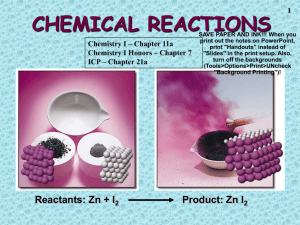
Intro to Chemical Equations and Reactions Name ____________________________ Chemical Equations use symbols and formulas to how substances change during a reaction: Reactants: The elements or compounds that we start with. Products: The elements or compounds that are produced in a chemical reaction. Law of Conservation of Matter (Mass): Equations are balanced to uphold this law. Atoms are neither created nor destroyed during ordinary chemical change. The atoms are simply rearranged. The total number of atoms before the reaction is equal to the total number of atoms after the reaction. REVIEW OF FORMULAS – Coefficients and Subscripts Formula How many of each type of atom in ONE molecule? How many of the whole Molecule? How many total Na2O 2Na2O 4Na2O S’MORES FORMULAS: 1. Write the formula for one s’more: _____________________ 2. Write the formula for two s’mores: _______________________ On your formula above, draw a square around the coefficient. Draw a circle around the subscripts. 3. If you have two s’mores how many of each type of “atom” do you have? _____ Gc _____ M _____Cp 4. Describe in words EVERYTHING a person can tell from this formula: 3Gc 2MCp3 5. Complete chemical equation for the making of a s’more. The reactants go on the left and the product on the right. _______ Gc + _______ M + _______ Cp __________________ 6. How do s’mores relate to chemical formulas? (Use complete sentences.) Balancing Chemical Equations: Equations are balanced to uphold the Law of Conservation of Matter. To balance a chemical equation: First, if the equation is not complete, write out the correct formulas… 1. Use charges 2. Know the 7 Diatomic Elements: Make sure you know which elements are diatomic so you can write the correct equation. Diatomic elements are elements so reactive that they react with themselves H2 , N2 , O2 , F2 , Cl2 , Br2 , I2 Second, add coefficients to make sure each element total on the reactant side is equal to element total on the product side. (Do not change subscripts when balancing!) Example: Write the equation for hydrogen and oxygen reacting to form water. Make a s’more and then balance these equations! Balancing – These start easier and get harder as you go. REMEMBER: Do not add/change subscripts! 1. Ca 2. NaCl 3. Mg 4. WO 5. CS2 + O2 + + + CaO Na + Cl2 HCl MgCl2 H2 W + H2O CO2 + SO2 O2 6. HCl + NaOH 7. Na2O + H2O + NaCl NaOH H2 + H2O 8. CaC2 + H2O Ca(OH)2 + C2H2 9. CO2 + H2O C6H12O6 + O2