1 - Unit #1-0
advertisement

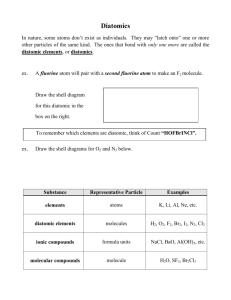
1. According to the HONC Rule, how many covalent bonds form around nitrogen? 1. ? one 2. ? two 3. ? three 4. ? four 5. 2. Which of the following is an acceptable Lewis Structure for the diatomic nitrogen molecule? 1. ? 2. ? 3. ? 4. ? 5. 3. In the correct Lewis structure for the methane (CH4) molecule, how many unshared electron pairs surround the carbon? 1. ? 0 2. ? 4 3. ? 2 4. ? 8 5. 4. In the correct Lewis structure for water, how many unshared pairs of electrons will oxygen have? 1. ? 3 2. ? 4 3. ? 1 4. ? 2 5. 5. Which of the following is an acceptable Lewis structure for chloromethane (CH3Cl)? 1. ? 1 6. 7. 8. 9. 2. ? 3. ? 4. ? 5. When compared to single bonds, double bonds are generally: 1. ? longer and stronger 2. ? shorter and weaker 3. ? longer and weaker 4. ? shorter and stronger 5. Which of the diatomic elements has a double bond between its atoms? 1. ? nitrogen 2. ? flourine 3. ? oxygen 4. ? hydrogen 5. The seven elements that occur as diatomic elements are: 1. ? H2, N2, O2, F2, Cl2, Br2, I2 2. ? H2, N2, O2, He2, Ne2, Cl2, Br2 3. ? H2, N2, O2, He2, Ne2, C2, Na2 4. ? Fe2, Rn2, O2, He2, Ne2, C2, Br2 5. In a diatomic molecule of an element, the bond between the atoms must be: 1. ? metallic 2. ? polar covalent 3. ? nonpolar covalent 4. ? ionic 2 10. In the ionic compound magnesium fluoride, what is the ratio of the two elements necessary so that each element obtains its octet from the transfer of electrons? 1. ? 1 magnesium : 1 fluorine 2. ? 1 magnesium : 2 fluorine 3. ? 2 magnesium : 1 fluorine 4. ? 3 magnesium : 1 fluorine 5. 11. How many sodium atoms are needed to provide the electrons necessary to complete the valence octet of an oxygen atom? 1. ? four sodium atoms 2. ? one sodium atom 3. ? two sodium atoms 4. ? three sodium atoms 5. 12. In the sodium chloride crystal lattice, each positively charged ion is surrounded by how many ions of opposite charge? 1. ? 8 2. ? 6 3. ? 2 4. ? 4 5. 13. Ionic compounds generally form: 1. ? gases 2. ? liquids 3. ? crystals 4. ? molecules 3