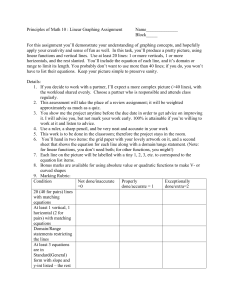
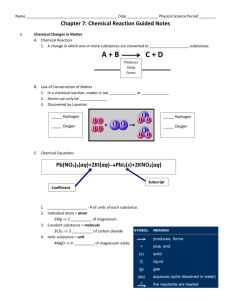
Balancing Reactions Quiz /14
advertisement

Name: Date: Period: Chemistry 11 Chapter 4 Outline Chapter 4 Expressing and Measuring Chemical Change By the end of this chapter, you should be able to do the following: 4.1 Writing and Balancing Chemical Equations – The magic of chemistry Apply the law of conservation of mass to balance formula equations a. b. c. d. Chemical reactions run our world The use of chemical shorthand Balancing chemical equations Keeping your balance – helpful hints 4.2 Classifying reactions and predicting products Explain chemical reactions in terms of the rearrangement of the atoms as bonds are broken and new bonds are formed Devise balanced equations for various chemical reactions a. b. c. d. e. f. Synthesis Decomposition Combustion Single replacement Double replacement Neutralization i. Acid + Base ii. Acid + carbonate iii. NH4 + Base 4.4 Energy Changes associated with Chem. Change Describe reactions in terms of energy changes a. Sources of Energy b. Energy in chemical bonds c. Thermochemical equations vs ΔH notation 4.5 Calculating with Chemical Change – Stoichiometry Perform stoichiometric calculations involving chemical reactions a. b. c. d. Moles to moles – application of the mole ratio Mass to mass – the molar mass revisited Gases – converting volume to volume Solutions – using molarity in volume conversions 4.6 Stoichiometry in the real world - Excess/Limiting amounts, % Yield, Impurities Perform stoichiometric calculations involving chemical reactions a. Excess and limiting – another issue to consider b. Percentage purity – impure reactants c. Percentage yield – incomplete reactions