Energy Types
advertisement
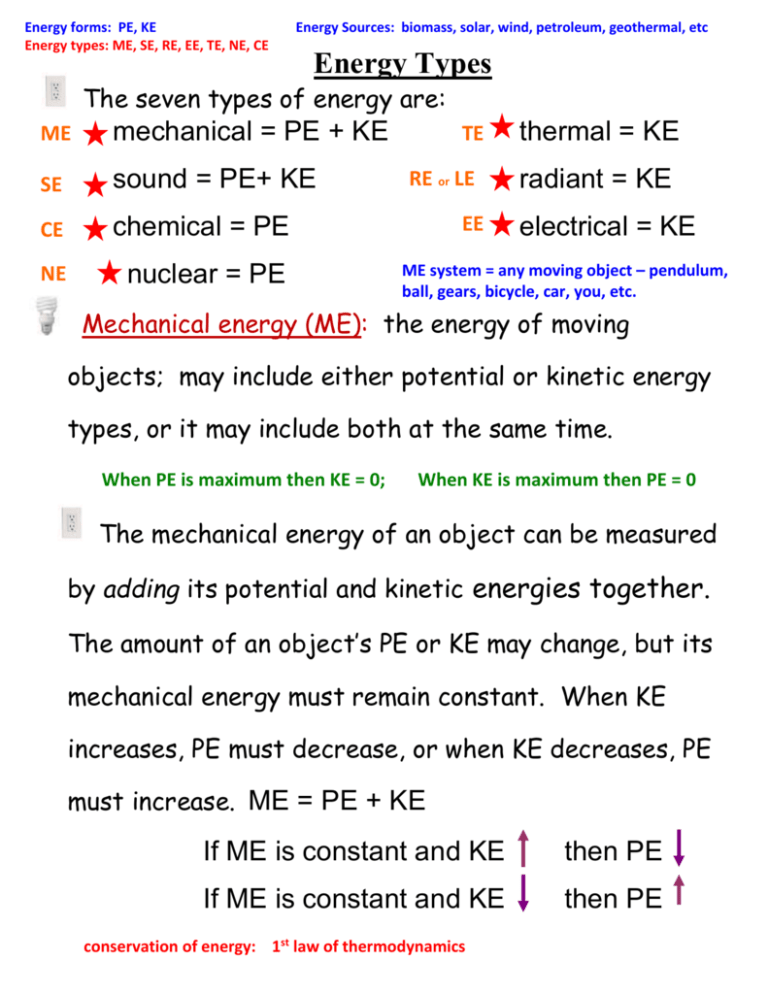
Energy forms: PE, KE Energy types: ME, SE, RE, EE, TE, NE, CE Energy Sources: biomass, solar, wind, petroleum, geothermal, etc Energy Types The seven types of energy are: ME mechanical = PE + KE SE sound = PE+ KE CE chemical = PE NE nuclear = PE TE RE or LE EE thermal = KE radiant = KE electrical = KE ME system = any moving object – pendulum, ball, gears, bicycle, car, you, etc. Mechanical energy (ME): the energy of moving objects; may include either potential or kinetic energy types, or it may include both at the same time. When PE is maximum then KE = 0; When KE is maximum then PE = 0 The mechanical energy of an object can be measured by adding its potential and kinetic energies together. The amount of an object’s PE or KE may change, but its mechanical energy must remain constant. When KE increases, PE must decrease, or when KE decreases, PE must increase. ME = PE + KE If ME is constant and KE then PE If ME is constant and KE then PE conservation of energy: 1st law of thermodynamics Energy Types – 2 A pendulum clock is a compound machine that uses stored energy to do work. A spring stores energy, and with each swing, some of the PE is converted to KE to move the hands. At the top of the swing PE is greatest and KE = 0. At the bottom of the swing, KE is the greatest and PE = 0. At any time, the PE and KE can be added to find the total energy of the system. The If the KE = 200J and increases, and the PE = 200J, what is the ME? Where is it in its swing? _____ _____ same concept applies with stretching materials like PE = Max PE = Max springs and rubber bands. In a pendulum if the ME = 200J and the KE = 150J and decreasing, what is the PE and where is it in its swing? _____ _____ A E B C KE = Max D KE – closely spaced particles push PE – widely spaced particles Sound = compression wave = P wave, like dominoes Why is there no sound in outer space? Energy Types – 3 speed of sound: air 68°F = 1130 ft/s, 770 mph; liquid water = 5000 ft/s, 3409 mph steel = 20,000 ft/s, 13,636 mph Sound energy (SE): Vibrating particles transmit their KE to the resting PE particle next to it. PE and KE are determined by the spacing or position of the particles. SE = KE + PE Sound must have a medium – matter that carries an energy wave. Polymer = chains of molecules = plastics, fuels Chemical energy (CE) is the energy that holds atoms in molecules together. It is a form of potential energy. Larger molecules have more chemical energy than smaller ones. Food and fuel are molecules that break apart easy, releasing their energy. All stuff has chemical energy! chemical PE changes into thermal KE + mechanical… Examples: sugar (carbohydrates), gasoline (hydrocarbons), ATP (cells), acids/bases - batteries, any type or fuel or food, all molecules!!! Carbohydrates: sugar = C6H12O6 carbs = long sugar chains fats = long carb chains Hydrocarbons: CH4 methane C3H8 propane C7 H16 gasoline C12 diesel/fuel oil C16 lubricating oil C20+ asphalt/tar/wax Most oil/petroleum is turned into plastic not gasoline! Matter is never lost! It goes somewhere, even if you can’t see it!! Energy Forms - 4 Where does stuff go when it burns up? Chemical reactions (breaking of atomic bonds) such as burning gasoline or digesting food releases the stored chemical energy and changes it into another form of energy (usually heat) which leaves the system. Energy is lost but total mass always stays the same. Combustion (burning) changes the size and shape but the total mass is exactly the same before and after. 1st LAW Thermodynamics Law of Conservation of Mass: Mass (matter) cannot be created or destroyed. mass in (reactants) = mass out (products) peak oil - point at which the earth's supply of oil will no longer be able to meet our energy needs. Oil is not a renewable energy source. It will be exhausted at some point in the future. ANYTHING INTO OIL? Thermal Depolymerization? ANWR: Arctic National Wildlife Refuge Should we drill for oil there? Neutral carbon sink = no additional carbon put into the atmosphere! Difference between CE and NE? CE = molecules; NE = protons and neutrons; NE = most energy of all types Energy Types - 5 Nuclear energy (NE): the energy that holds protons and neutrons together inside the nucleus of an atom; PE; the most powerful energy source every discovered; produced in two ways: Nuclear fusion: energy stored in hydrogen atoms join to form helium releasing large amounts of electromagnetic energy; our sun Nuclear fission: the nuclei of large atoms are split converting the potential nuclear energy into kinetic electromagnetic energy Nuclear Reactors? Nuclear waste/pollution? no fuel is burned so no air pollution is produced carbon footprint? NONE! Water is produced! Uranium fuel rods last about 2-3 years; must be stored until radioactivity is lost after about ten years – metal recycled! Nuclear energy is clean and environmentally friendly!!! Safety? Compare driving vs. flying; nuclear submarines? Japanese earthquake? Chernobyl? Three Mile island? Nuclear energy is very safe! Nonrenewable; 230 years to 1000 yrs? One pellet the size of your fingertip produces the same amount of energy as 150 gallons of oil 10 million times as much heat as coal cold metal versus warm plastic? endothermic versus exothermic Energy Types - 6 Thermal energy (TE) is the total kinetic energy of the particles that make an object. It is the vibration of all the atoms or molecules within an object. All atoms and molecules are in motion at all times. At room temperature, atoms jiggle at about 1000 mph. Thermal energy is measured in joules. Temperature is a measure of the average kinetic energy of the particles in an object. It is measured in °F or °C or K. It is the intensity of the motion of the particles. At higher temperatures, particles of matter move faster and have more kinetic energy. At lower temperatures, particles move slower and have lower kinetic energy. What is the Kelvin scale? What is absolute zero? Energy Types - 7 Temperature does not depend on the size or type of object. For example, the temperature of a small cup of water might be the same as the temperature of a large tub of water, but the tub of water has more thermal energy because it has more water. High up in the atmosphere, the temperature increases to over 3600F because gas molecules absorb solar wind and move very rapidly. But since the molecules are spaced far apart, the thermal energy is low even though the temperature (degrees Fahrenheit or Celsius) is high. The international space station is here and does not burn up. Heat is not exactly the same as thermal energy. Heat is energy that is transferred or moving between substances due to a temperature difference between them. The amount of heat transferred by a substance depends on the speed and number of atoms or molecules in motion. The faster the atoms or molecules move, the higher the temperature, and the more atoms or molecules that are in motion and the greater the amount of heat they transfer. According to the second law of thermodynamics, heat always flows from areas of high temperature to areas of low temperature. Energy Types - 8 Friction is the thermal energy that opposes motion. It includes the heat given off when things touch. It is the energy transfer or change from mechanical energy to thermal energy. The four states of matter (solid, liquid, gas, plasma) are scientifically defined by the ratio of a matter’s used atomic bonds to the number of bonds possible. Matter states are more easily and commonly defined: the amount of chemical and thermal energy the space between the matter’s molecules Solid: low energy; molecules are close together; molecules are not moving fast enough to overcome the attraction of other molecules. Gas: high amount of energy - too much energy to allow the atoms to attract and stick much; molecules move fast and are far apart Liquid: in between a solid and gas; molecules move fast enough to overcome some of the attraction between them; may change shape but not volume Plasma: super high energy; atoms are stripped of their electrons so they cannot bond together; sun, lightning Energy Types - 9 Electrical energy (EE) is the movement of electrons through matter. It is kinetic energy. When electrons are knocked out of their orbit, electricity is created. There are two kinds of electricity - static and current. Static electricity is the random and scattered movement of electrons in an area where they spread out. Every time two things touch, they trade electrons and become ions with either a positive or negative charge. No work is done in static electricity because the electrons are scattered. Sometimes the electrons pile up and form a charge until they find a conducting path. Lightning is static electricity. Current electricity is the continuous flow of electrons along a directed path called a circuit. Since we control where the electrons go, work is done. Current always flows from a place where there are many electrons (negative charge) to a place where there are fewer electrons (positive charge). This movement creates a loop. Current always flow from negative (-) to positive (+). What happens to the magical mystery missing sock in the cloths dryer? lightning? electrical safety? 50,000 Volts versus 0.1 Amps – heart attack/death; 0.018A breathing difficulty/suffocation; 0.0001A human perception Difference between light and sound? Energy Types - 10 Radiant energy (RE) is the energy from electromagnetic waves. Electromagnetism is a wave of pure energy defined by its wavelength and frequency (the amount of energy in a space). The electromagnetic spectrum includes radio waves, TV waves, infrared (heat), visible light, ultra violet, X-rays, gamma rays. Radiant energy travels at about 186,000 miles per second in a vacuum (absence of matter - space). spectrum = range of energy types Energy Types - 11 Each of the seven forms of energy can be changed into any one of the other energy forms. A change in energy form is called an energy conversion. All energy comes from other forms of energy. This is the first law of thermodynamics. Examples: light energy from the sun can be converted into heat energy by the ground and into chemical energy stored in sugar by photosynthesis in plants; a battery may convert chemical energy into electrical energy; a stereo may convert electrical energy into sound energy using the speakers; a combustion engine converts chemical energy from the gasoline into mechanical energy which pushes the piston down. Energy conversions often involve the changing or movement of matter. There are three types of objects or areas where matter and energy conversions take place (isolated, closed, open). A system is a place or object where matter and energy exist and energy conversions take place over a certain period of time. Systems often exist inside other systems. For example: nervous, cardiovascular, respiratory, muscle, and skeletal systems all operate together inside the human body system. A machine is a man-made system that makes work easier by changing the size or direction of the force. Energy Types - 12 Systems try to control the type of energy conversions that happen in order for them to do work. But, not all the energy in a conversion in a system becomes useful energy. Any time that things touch, they trade energy and heat is lost which is wasted energy. In totally 100% efficient systems, all the energy put in does exactly what was intended and is useful. Because parts in a system must touch and trade heat, no system can ever be 100% efficient. THREE SYSTEMS: Isolated system: an exchange or transfer of matter and energy only within the system and none outside to the surrounding - everything stays in and stays out. these are impossible because they violate the first law of thermodynamics - everything must touch and trade heat so nothing can be totally isolated and 100% efficient! There are no isolated systems inside the universe; the only known isolated system is the universe itself as whole. The main goal of all engineering is to get as close as possible to an isolated system or 100% efficient. Energy Types - 13 Closed system: an exchange or transfer of matter and energy within the system, and where there is no transfer of matter outside, but there is some exchange of energy outside the system - heat is given off. they will eventually run out of energy or fuel. most created machines and most natural or biological systems are considered to be closed at least for periods of time. Examples: a bouncy ball, a battery circuit, cars and airplanes (after refueling not during), animals (not including breathing, after eating a meal not during). Open system: transfer of both matter and energy both inside and outside different systems - matter and energy enter and leave the system. Most machines are not open because there would need to be constant change in either the amount of work being done or a constant refueling/feeding process; resources are renewed and exchanged on a regular basis. Examples: refrigerator or electric appliances – something constantly plugged in, volcanoes, hurricanes, tornadoes, weather fronts, solar system, ecosystems, water cycle. PEAK OIL World discovery of oil peaked in the 1960s, and has declined since then. If the 40 year cycle seen in the US holds true for world oil production, that puts global peak oil production, right about now; after which oil becomes less available, and more expensive. Today we consume around 4 times as much oil as we discover. If we apply Hubbert's Peak to world oil production we estimate that approximately half of all oil that will be recovered, has been recovered, and oil production may reach a peak in the near future, or perhaps already has. http://peakoil.com/what-is-peak-oil/ HYDROCARBONS In a 42 gallon barrel of crude oil 20 gal 0.2 gal 4 gal 9 gal 0.5 gal 2 gal 6 gal Equivalent Resistance: 1/Req = 1/R1 + 1/R2 + 1/R3 1/Req = 1/(5.0 ) + 1/(7.0 ) + 1/(12 1/Req = 0.42619 -1 Req = 1 / (0.42619 -1) Req = 2.3 ) ELECTRICAL SAFETY As shown in the chart, shock is relatively more severe as the current rises. For currents above 10 milliamps, muscular contractions are so strong that the victim cannot let go of the wire that is shocking him. At values as low as 20 milliamps, breathing becomes labored, finally ceasing completely even at values below 75 milliamps. As the current approaches 100 milliamps, ventricular fibrillation of the heart occurs - an uncoordinated twitching of the walls of the heart's ventricles which results in death. Above 200 milliamps, the muscular contractions are so severe that the heart is forcibly clamped during the shock. This clamping protects the heart from going into ventricular fibrillation, and the victim's chances for survival are good.