Thermal Physics Revision Q`s HL - MS - SJHS-IB
advertisement
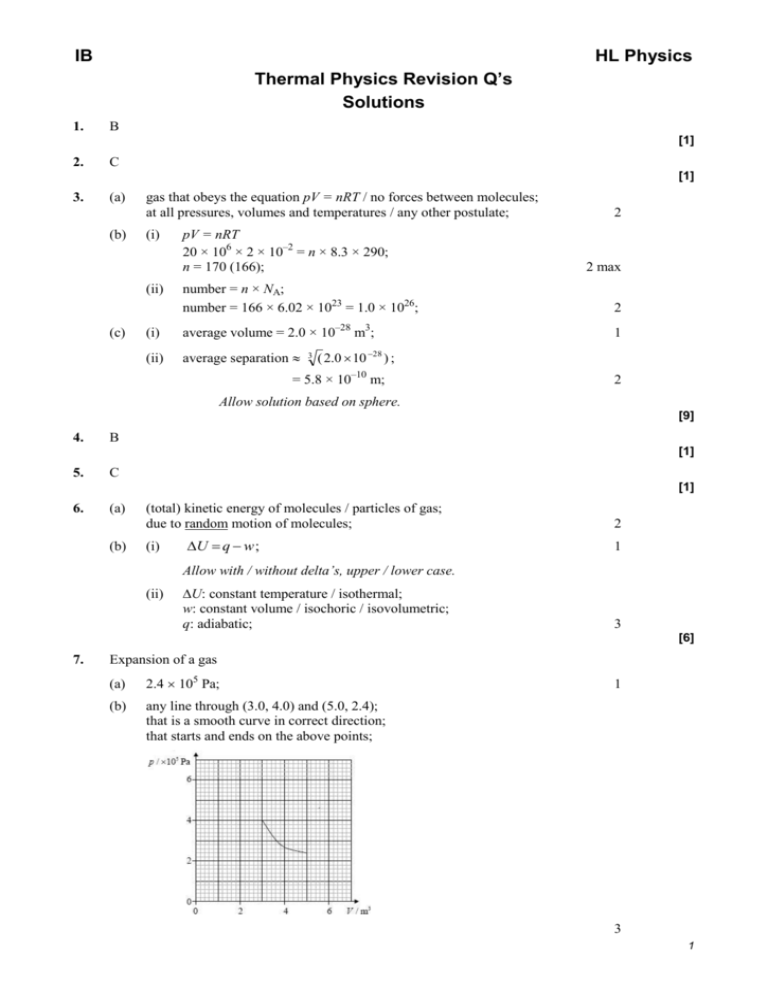
IB HL Physics Thermal Physics Revision Q’s Solutions 1. B [1] 2. C [1] 3. (a) (b) gas that obeys the equation pV = nRT / no forces between molecules; at all pressures, volumes and temperatures / any other postulate; (i) (ii) (c) pV = nRT 20 × 106 × 2 × 10–2 = n × 8.3 × 290; n = 170 (166); 2 2 max number = n × NA; number = 166 × 6.02 × 1023 = 1.0 × 1026; 2 (i) average volume = 2.0 × 10–28 m3; 1 (ii) average separation 3 ( 2.0 10 28 ) ; = 5.8 × 10–10 m; 2 Allow solution based on sphere. [9] 4. B [1] 5. C [1] 6. (a) (b) (total) kinetic energy of molecules / particles of gas; due to random motion of molecules; (i) U q w ; 2 1 Allow with / without delta’s, upper / lower case. (ii) U: constant temperature / isothermal; w: constant volume / isochoric / isovolumetric; q: adiabatic; 3 [6] 7. Expansion of a gas (a) 2.4 105 Pa; (b) any line through (3.0, 4.0) and (5.0, 2.4); that is a smooth curve in correct direction; that starts and ends on the above points; 1 3 1 (c) (i) (ii) work done = area under line / curve / graph; to get 6.1 105 J; Accept 5.5 6.7 105 J. 2 work done would be less as adiabatic line is steeper than isothermal line / OWTTE; or: no energy / heat has to be transferred to the surroundings to maintain constant temperature / OWTTE; 1 [7] 8. (a) (b) (c) (d) V1 V 2 ; T1 T2 V 6 T2 2 T1 300 = 900 K; V1 2 use of W(= pV = 12 × 105 × 4.0 × 10–3 = 4.8 × 103 J) = 4.8 kJ; Q = (4.8 kJ + 7.2 kJ) = 12 kJ; Award full marks for correct answer without work shown. the (magnitude of the) temperature change is the same; the gas is ideal and so the change in internal energy depends only on temperature; (i) 2 2 2 for the full cycle ∆U = 0; therefore the net work is 4.8 – 2.6 = 2.2 kJ; or net thermal energy in is 12 kJ and net thermal energy out is 7.2 + 2.6 = 9.8 kJ; so work done is 12 – 9.8 = 2.2 kJ; or (ii) work is area in loop; area = 4.8 – 2.6 = 2.2 kJ; 2 efficiency is 2 .2 ; 12 = 0.18 /18 %; 2 [10] 2