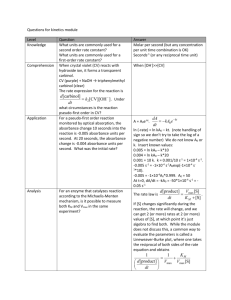
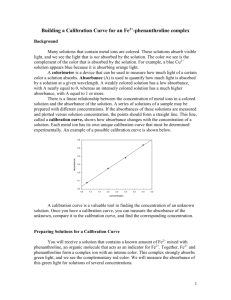
Beer`s Law Lab
advertisement
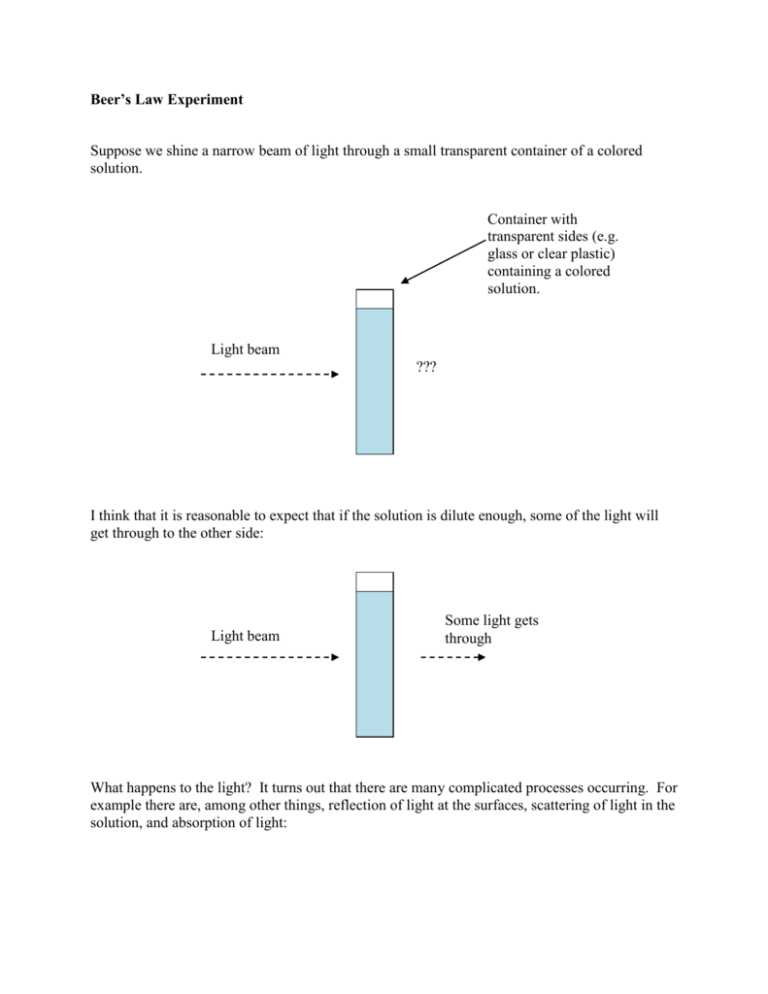
Beer’s Law Experiment Suppose we shine a narrow beam of light through a small transparent container of a colored solution. Container with transparent sides (e.g. glass or clear plastic) containing a colored solution. Light beam ??? I think that it is reasonable to expect that if the solution is dilute enough, some of the light will get through to the other side: Light beam Some light gets through What happens to the light? It turns out that there are many complicated processes occurring. For example there are, among other things, reflection of light at the surfaces, scattering of light in the solution, and absorption of light: Scattering Light beam Reflection at the surfaces However, we can control from at least some of these complicated interactions by using two identical containers (or even better, the same container). Controlling in this way, we can ask interesting questions about the amount of light that gets through. For example: How does the amount of light passing through vary when we change the thickness through which the light passes? How does the amount of light passing through vary when we change the concentration of the solution? These questions were investigated by scientists in 1700’s and 1800’s (Bouger in 1729, Lambert in 1760, and Beer 1852) and what they discovered is extremely useful to this day. In particular the answer to the second question is extensively used in chemistry and biochemistry. For us, it is an excellent example of linear modeling. What Bouger and Lambert discovered was that if a quantity called the absorbance of the light in a fairly natural way (described below), there is an approximately linear relationship between absorbance and pathlength: What Beer discovered was that, at low concentrations, there is an approximately linear relationship between absorbance and concentration: The usefulness of these relationships is that one can find determine the concentrations of solutions whose concentrations are unknown by measuring the amount of light passing through them (once one knows the equation of the line for the particular type of solution). We will see how this process works below. Technical aside: What is absorbance? Intensity of light is generally measured in units of power per area (watts per square meter for example). This concept corresponds exactly to our feeling of the intensity of the light of sun on a sunny day. We can design instruments to measure intensity of light. Thus we can measure the intensity of light before and after the light passes through the solution: Measure Intensity Ibefore Measure Intensity Iafter For solutions, the absorbance is defined to be the negative of the base 10 logarithm of ratio of intensity after to intensity before, i.e., A log10 Iafter I before For us, what is important is that this quantity called absorbance is measurable with instruments and corresponds in a natural way to what we think of as absorbance (namely a high absorbance means not much light gets through while a low absorbance means a lot of light gets through, an absorbance of 0 means no light is absorbed at all: Iafter Ibefore ). Activity In this activity you will produce the absorbance curve for a food dye solution by using a simple colorimeter which measures the absorbance. You will find the regression line that models the relationship and determine the concentration of an unknown solution. Materials Colorimeter with interfacing cable TI-84 calculator (or computer with LabPro software installed) 1 cuvette with distilled water (the blank) 5 cuvettes with standards for food dye solution Cuvette with an unknown either A or B or C. Steps 1. Plug the colorimeter into the calculator. Normally, the EasyData program will come up automatically and detect the colorimeter. If not, click on Apps and arrow down to EasyData. The interface software should give the absorbance readings. Make sure that the colorimeter is set to 635 nm. 635nm is the wavelength of the light being used for the measurements. Light with wavelength of 635 nm is red. The colorimeter is capable of using four different wavelengths. More sophisticated instruments, generally called spectrophometers, are capable of using hundreds of wavelengths. When using a spectrophometer, one usually chooses the wavelength with the highest absorbance. Of the four wavelengths available to us, 635 nm gives the highest absorbance, and 635 nm is rather close the maximum absorbance for this solution. 2. Measure the absorbance for each of the solutions labeled 1 through 5 as follows. Put the blank in first. Press Cal and wait until the instruments zeroes out. Then put the cuvette that you want to measure into the colorimeter and take the measurement. Repeat this procedure for each of the five standards (i.e., put the blank in each time and zero it out). Make sure that the hatched sides of the cuvette are always parallel to the instrument (when looking at the instrument straight on). In general, try not to touch the clear sides of the cuvettes. Record your data in your calculator as follows. You also can use Excel if it is available to you. Solution 0 1 2 3 4 5 Enter this in calculator Concentration (mol L-1) Absorbance L1 L2 0.000 0 1.07835E-06 2.15670E-06 3.23505E-06 4.31340E-06 5.39175E-06 3. Make a scatter plot of the data with concentration on the x-axis and absorbance on the yaxis. Add a best fit regression line. Record the equation of the trendline and the Rsquared value. 4. You will have been given an unknown (A or B or C). Find the absorbance of the unknown using the colorimeter. 5. Using your regression line, estimate the concentration of the unknown. What you should hand in 1. 2. 3. 4. 5. Your data table Your scatter plot of the data with the linear trendline. The equation of your trendline. Your estimated concentration of your unknown A or B or C. Answer the following question. You will notice that there is variance in the data. For one thing, the data doesn’t fall exactly on a line. If you make multiple measurements with the same cuvette over time, you will get slightly different readings. Name at least two sources of variation in this experiment.