Experiment33
advertisement
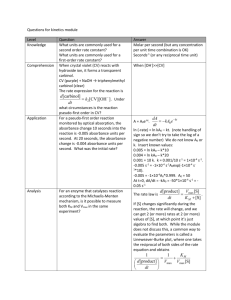
Experiment 33: Colorimetric determination of iron PURPOSE To become aquainted with the principles of colorimetric analysis. THEORY • When mixed with phenanthroline , Fe2+ ions reacts to form an orange red complex • The color of the solution is directly proportional to the concentration of Fe3+ ions present. The concentration of these ions can be determined by measuring the absorbance of a unknown Fe2+ complex solution and comparing it with the absorbance of a solution of known concentration. • Colorimeters measure the amount of light that is transmitted or absorbed by a solution. A description of how they work can be found in your text book • Fe3+(aq) needs to be reduced to Fe2+ by adding hydroxlyamine SAFETY PROCEDURES • Follow all instructions for using the equipment in this activity. • 2. Wear safety glasses and a laboratory coat for this experiment. • 3. Do not throw any waste down the sink. Part 1. Preparation of calibration curve • Pipet 1.00, 2.00, 3.00, 4.00, and 5.00 ml of standard Fe solution into a 50 ml volumetric flasks 1 – 5 respectively • Add to each flask – 1ml of 1M ammonium acetate – 1ml of 10% hydroxylamine – 10 ml of .30% phenanthroline – Dilute the rest to 50 ml with water Part 1. Preparation of calibration curve • Mix well • Let sit for 40 minutes for the color to develop • • • • Clean six cuvettes Add water to one cuvette ½ way and calibrate spectrophotometer to 510 nm Fill the other 5 cuvettes ½ way with a solution of increasing concentration Record absorbance Part 1. calibration curve • graph concentration of Fe+2 complex (mg/ml) vs absorbance • Absorbance on y axis and concentration on x as seen on page 425 concentration Absorbance of Fe+2 complex (mg/ml) 1 2 .001 .15 calibration curve Absorbance • Prepare calibration curve by plotting absorbance vs concentration y = 59.524x 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 Y-Values Linear (Y-Values) 0 0.005 0.01 Fe +2complex mg/ml x 10 -3 Part B: Determination of Fe+3 complex Create Fe +2 ion • Weigh 0.1 g to 4 sig figs of Fe unknown into a 50 ml volumetric flasks • Add – 5 drop of 6 M sulfuric acid – Dilute the rest to 50 ml with water – Transfer to a 125 erlenmeyer flask Part B: Determination of Fe+3 complex Convert Fe +2 ion to Fe+3 complex • Pipet 1ml of Fe +2 ion from the erlenmeyer flask into three 50 ml volumetric flasks • Add to each flask – – – – 1ml of 1M ammonium acetate 1ml of 10% hydroxylamine 10 ml of .30% phenanthroline Dilute the rest to 50 ml with water Part B: Determination of Fe+3 complex Mix well Let sit for 40 minutes for the color to develop • • • • Clean 3 cuvettes Fill the 3 cuvettes ½ way with each Fe+3 complex solution Record absorbance at 510 nm Using the absorbance determine concentration of Fe+2 complex (mg/ml) by extrapolating from your graph