downloaded here
advertisement
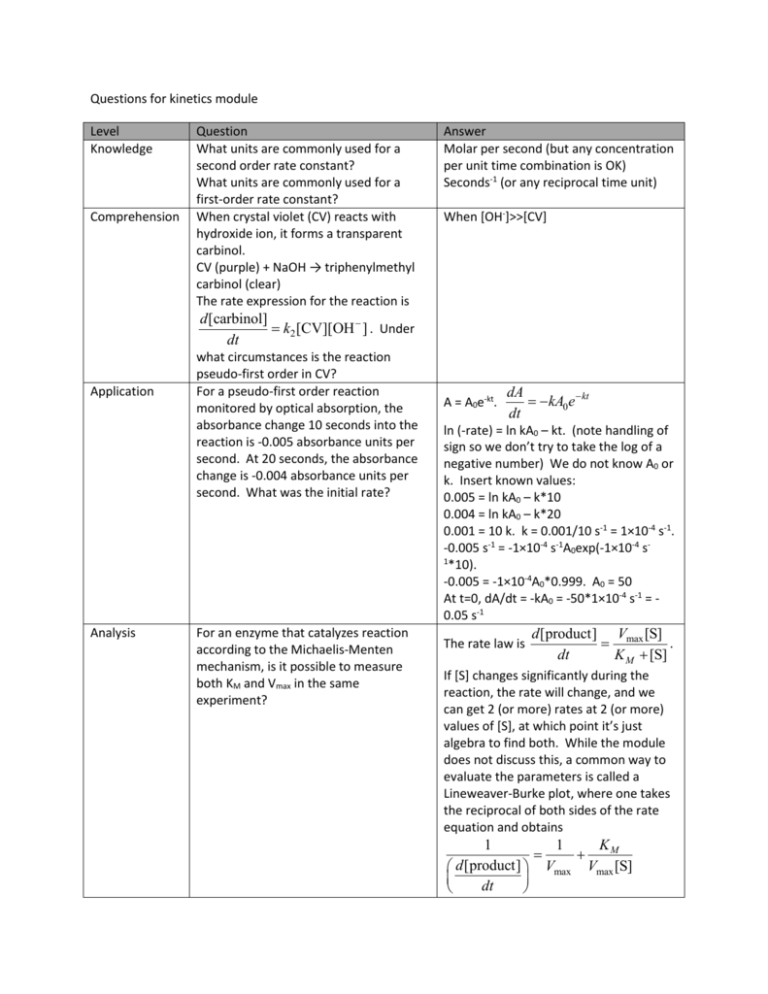
Questions for kinetics module Level Knowledge Comprehension Question What units are commonly used for a second order rate constant? What units are commonly used for a first-order rate constant? When crystal violet (CV) reacts with hydroxide ion, it forms a transparent carbinol. CV (purple) + NaOH → triphenylmethyl carbinol (clear) The rate expression for the reaction is Answer Molar per second (but any concentration per unit time combination is OK) Seconds-1 (or any reciprocal time unit) When [OH-]>>[CV] d [carbinol] k2 [CV][OH ] . Under dt Application Analysis what circumstances is the reaction pseudo-first order in CV? For a pseudo-first order reaction monitored by optical absorption, the absorbance change 10 seconds into the reaction is -0.005 absorbance units per second. At 20 seconds, the absorbance change is -0.004 absorbance units per second. What was the initial rate? For an enzyme that catalyzes reaction according to the Michaelis-Menten mechanism, is it possible to measure both KM and Vmax in the same experiment? A = A0e-kt. dA kA0 e kt dt ln (-rate) = ln kA0 – kt. (note handling of sign so we don’t try to take the log of a negative number) We do not know A0 or k. Insert known values: 0.005 = ln kA0 – k*10 0.004 = ln kA0 – k*20 0.001 = 10 k. k = 0.001/10 s-1 = 1×10-4 s-1. -0.005 s-1 = -1×10-4 s-1A0exp(-1×10-4 s1 *10). -0.005 = -1×10-4A0*0.999. A0 = 50 At t=0, dA/dt = -kA0 = -50*1×10-4 s-1 = 0.05 s-1 The rate law is d [product] Vmax [S] . dt K M [S] If [S] changes significantly during the reaction, the rate will change, and we can get 2 (or more) rates at 2 (or more) values of [S], at which point it’s just algebra to find both. While the module does not discuss this, a common way to evaluate the parameters is called a Lineweaver-Burke plot, where one takes the reciprocal of both sides of the rate equation and obtains KM 1 1 d [product] Vmax Vmax [S] dt Synthesis Evaluation Both PO43- and SiO44- can react with acidified molybdate to make yellow heteropolymolybdate complexes. If one starts with excess molybdate, what would you expect the rate of absorbance change to be, starting with a mixture of silicate and phosphate? How would you determine if your rate law corresponded to experimental reality? For a particular pH, it takes 15 minutes for phosphate reacting with molybdate to reach stable absorbance (where dA/dt due to noise exceed dA/dt due to reaction). What questions would you ask to decide whether to use reaction rate or equilibrium absorbance to measure [PO43-]? One would expect the reaction to be pseudo-first order in each reactant. For absorbance at some appropriate wavelength, dA k P [PO34 ] k S [SiO 44 ] dt One might make a set of phosphate solutions, a set of silicate solutions, and a set of solutions with both, measure dA/dt, and determine the two rate constants and if those constants held in the mixture of the two ions. a) Is there a background absorbance? If so, prefer kinetics. b) Is detection limit for kinetics measurement adequate? c) Is selectivity of equilibrium measurement adequate? d) Does either method have adequate precision? e) Is the faster experiment time for kinetics (< 15 minutes) useful?