Kinetics of the Decomposition of Crystal Violet Indicator
advertisement
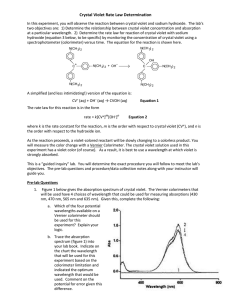
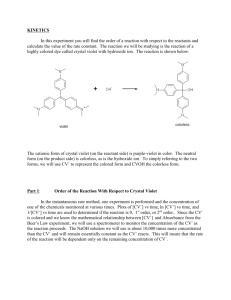
Kinetics of the Decomposition of Crystal Violet Indicator Purpose To conduct an experiment that will collect data and determine the order of a chemical reaction. Concept Kinetics is the branch of chemistry that describes the rates of reactions and their mechanisms. Application is left to the student Safety H Crystal Violet 1 0.10M NaOH 2 F 0 0 R 1 2 Special Stains Caustic Setup Procedure.1 Set up the CBL-2 with a graphing calculator and a colorimeter in channel 1. Setup Procedure.2 Calibrate the colorimeter Set the dial to 0%T; Calibrate point 1 at 0. Place a cuvette with dH2O in the colorimeter. Setup Procedure.3 Set the dial to 656nm (Green); Calibrate point 2 at 100. Return to the main screen. Setup Procedure.4 Set the mode to time graph Use these values to run the experiment. Setup Procedure.5 Return to the main screen Reaction Procedure.1 Begin the reaction. • Add 10 ml of NaOH and the 80 ml of distilled water to a clean beaker and stir. 10 ml of CV 80 ml of dH2O 10 ml of NaOH Reaction Procedure.2 • Add 10 ml of Crystal Violet solution to the beaker and stir. Reaction Procedure.3 • Obtain an aliquot of the reaction and place it in a cuvette. • Insert the cuvette into the colorimeter and start recording the data with the CBL-2. Start Reaction Procedure.4 The calculator will display the data as it is collected and graphically track the progress of the reaction. Reaction Procedure.5 Once the CBL-2 has finished collecting data it will display a graph of the collected data. Reaction Procedure.6 Clean up all the glassware and the cuvette then return the equipment as directed. Rinse all glass ware with dH2O and dry before storing. Do not wipe the cuvette dry with any thing other than the optical wipes. Data In this experiment the data was automatically collected by the CBL-2 and stored in the graphing calculator. Nearly 200 data points were collected. For convenience they are not listed here. Data Analysis.1 The CBL-2 collected absorbance data. By using Beer’s Law we can conclude that absorbance is directly proportional to the concentration of the Crystal Violet indicator. Therefore absorbance data can be treated as concentration data. Data Analysis.2 In order to determine the order of the kinetics we need to evaluate how the concentration of Crystal Violet [CV] (absorbance data) changed with respect to time. Data Analysis.3 If the reaction is Zero Order then a graph of [CV] vs. Time will be linear. It’s not linear. Data Analysis.5 If the reaction is First Order then a graph of ln[CV] vs. Time will be linear. It’s Linear! Data Analysis.6 If the reaction is Second Order then a graph of 1/[CV] vs. Time will be linear. It’s not. Conclusion Summarize the lab procedures. Give the result(s). Determine source(s) of error. Describe an error propagation.