Ezetimibe Added to Statin Therapy After Acute Coronary Syndrome
advertisement

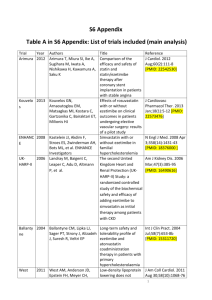
Ezetimibe Added to Statin Therapy After Acute Coronary Syndrome NEJM 2015; 372(25):2387-2397 Mentor: Dave L. Dixon, PharmD, AACC, FNLA, CDE, CLS, BCPS-AQ Cardiology Presented: September 25, 2015 Kyle D. Thorner, PharmD WakeMed Health & Hospitals Article Summary Background: Ezetimibe reduces serum LDL-cholesterol via inhibition of intestinal cholesterol absorption via the NiemannPick C1 transporter (NPC1). It is hypothesized that ezetimibe should provide cardiovascular benefit in addition to LDL cholesterol reduction. Mutations resulting in inactive or partially inactive NPC1 have been associated with a reduction in cardiovascular disease (CVD). However, previous trials have failed to show cardiovascular benefit beyond LDL cholesterol reduction and the SEAS trial introduced a potential cancer signal associated with ezetimibe. Objective: To evaluate the effect of ezetimibe combined with simvastatin, as compared with that of simvastatin alone, in stable patients who had an acute coronary syndrome and whose LDL cholesterol values were within guideline recommendations. Study Population: Results: - Baseline Characteristics: Average age 63.6 years, ~76% male, ~84% white, mean baseline LDL-C of 93.8 mg/dL, index event UA (~29%), NSTEMI (~47%), or STEMI (~24%). Baseline medications: statin (~35%), aspirin (~42%), post-ACS medications: aspirin (~97%), thienopyridine (~86%), BB (~87%), and ACEI (75%) - Median follow-up: 6 years - Total follow-up: 7 years Outcome LDL-C at 1 year (mg/dL) Primary composite (%) Nonfatal MI (%) Inclusion Criteria Age ≥ 50 years, hospitalization within previous 10 days for ACS, LDL cholesterol ≥ 50 mg/dL, LDL ≤125 mg/dL for patients naïve to lipid-lowering therapy, LDL ≤100 mg/dL for patients on lipid-lowering therapy Exclusion Criteria Planned CABG, CrCl < 30 ml/min, active liver disease, use of statin therapy with LDL-lowering potency greater than 40 mg of simvastatin Study Design: IMPROVE-IT was an international, multicenter, double-blind, placebo-controlled trial. Patients were randomized to receive simvastatin 40 mg once daily plus ezetimibe 10 mg daily or simvastatin 40 mg once daily plus placebo. Outcomes: - Primary: Composite of death from CVD, major coronary event (nonfatal myocardial infarction, unstable angina requiring hospitalization, or coronary revascularization after 30 days) or nonfatal stroke - Secondary: Various composite outcomes - Tertiary: Individual components of the composite outcomes - Safety: Liver enzyme levels, creatinine kinase levels, incidence of myopathy, incidence of rhabdomyolysis, gallbladder-related adverse events, and incidence of cancer Ischemic Stroke (%) ALT, AST or both ≥3 x ULN Rhabdomyolysis Myopathy Cancer *Yearly NNT = 350 - Simvastatin (n=9077) SimvastatinEzetimibe (n=9067) HR (95% CI) p-Value (NNT) 69.9 53.2 - <0.001 2742 (34.7) 2572 (32.7) 1083 (14.4) 945 (12.8) 297 (4.1) 236 (3.4) 0.936 (0.89-0.99) 0.87 (0.8-0.95) 0.79 (0.67-0.94) 0.016 (50)* 0.002 (63) 0.008 (143) 208 (2.3) 224 (2.5) - 0.43 18 (0.2) 10 (0.1) 732 (10.2) 13 (0.2) 15 (0.2) 748 (10.2) - 0.37 0.32 0.57 Subgroup Analysis: Significant difference observed in the primary endpoints for patients with diabetes (NNT=19) and patients age ≥75 (NNT=12) By the end of the study, 42% of patients in each study arm were no longer taking originally assigned treatment. Conclusions: The addition of ezetimibe to statin therapy in stable patients with recent ACS and who had LDL cholesterol levels within guideline recommendations further lowered the risk of cardiovascular events. Discussion: - First clinical trial to show cardiovascular benefit from the addition of a non-statin to statin therapy. Benefit seen without an increase in adverse events. - Utilization of moderate intensity statin for monotherapy arm does not reflect current practice. - The significant lowering of LDL cholesterol combined with reduction in cardiovascular endpoints, albeit modest, lends support to the LDL hypothesis, but does not disprove the statin hypothesis. Ezetimibe Added to Statin Therapy After Acute Coronary Syndrome NEJM 2015; 372(25):2387-2397 Mentor: Dave L. Dixon, PharmD, AACC, FNLA, CDE, CLS, BCPS-AQ Cardiology Presented: September 25, 2015 Questions: 1) Would you include ezetimibe as step therapy before a PCSK-9 inhibitor? After maximizing statin therapy, I would include the addition of ezetimibe ahead of PCSK-9 inhibitors for step therapy. However, there are several factors to consider. While IMPROVE-IT demonstrated significant LDL lowering and modest cardiovascular benefit with the addition of ezetimibe to simvastatin, PCSK-9 inhibitors have been shown to dramatically decrease LDL cholesterol by as much as 60% in in addition to statin therapy and provide benefit in cardiovascular outcomes as well. (1,2) Patient compliance may be higher with ezetimibe despite additional pill burden if patients are unwilling to subject themselves to injections. Financially, ezetimibe will eventually be the far less expensive option when generic versions become available in April 2017, which will include the combination of simvastatin and ezetimibe. (3) 2) What are your thoughts about the additional cost of ezetimibe? Is the higher cost worth the achieved outcomes? If a patient is able to tolerate high-intensity statin therapy, I do not think the additional cost of ezetimibe would make up for the residual benefit of adding ezetimibe. The PROVE-IT TIMI 22 study demonstrated the benefit of high intensity statin therapy compared to moderate intensity statin therapy in patients with recent ACS for reducing the primary endpoint of death, MI, unstable angina requiring hospitalization, stroke or revascularization. (4) Therefore, it is likely that a high intensity statin monotherapy arm would produce at least the same outcomes as the addition of ezetimibe added to moderate intensity statin therapy. The average wholesale prices (AWP) for a thirty-day supply of ezetimibe and ezetimibe/simvastatin are $283.85 and $281.26, respectively. (5) While this does represent a financial obstacle, the cardiovascular outcome benefit seen in IMPROVE-IT may justify the additional expense in patients only able to tolerate moderate-intensity statin therapy. Specifically, the addition of ezetimibe to statin therapy reduced non-fatal MI and ischemic stroke, which represent a substantial economic burden themselves. Lastly, generic versions of ezetimibe will be available in the near future. (3) Kyle D. Thorner, PharmD WakeMed Health & Hospitals 3) What are your thoughts regarding the pill burden due to the addition of ezetimibe? I believe that the pill burden with the addition of ezetimibe to statin therapy is negligible. Ezetimibe does not require more than once daily dosing and can be taken at the same time of day as the statin therapy without fear of any kind of drug interaction. Also, if an addition tablet was of concern, the combination tablet of simvastatin/ezetimibe is approximately the same price as the ezetimibe alone. (5) 4) Clarify what your opinion of the discontinuation rates seen in IMPROVE-IT? The discontinuation rate in IMPROVE-IT was substantial with 42% of patients in each arm not taking their originally assigned treatment by the end of the trial. (1) I do think this makes the interpretation of the trial’s results difficult. However, an ontreatment analysis of the IMPROVE-IT trial showed similar LDL-cholesterol lowering and the primary endpoint remained statistically significant, HR 0.92 (CI 0.868-0.983, p=0.012). (5) I think this report makes it easier to trust that the benefit seen in IMPROVE-IT was due to the addition of ezetimibe and not from other lipid-lowering regimens that patients may have been on throughout the trial. 5) Would you still add ezetimibe to a patient on atorvastatin 80mg who had an LDL > 70mg/dL? I am hesitant to recommend the addition of ezetimibe to highintensity statin therapy, like atorvastatin 80 mg, due to a lack of evidence for this practice and the additional financial burden that would be placed upon the patient. Utilizing the results of the PROVE-IT TIMI 22 study, which showed the benefit of high intensity statins over moderate-intensity statins in patients with recent ACS, it may be postulated that a monotherapy high intensity statin arm in IMPROVE-IT very well may have produced equal, if not improved, outcomes as the ezetimibe/simvastatin arm. (1,4) Therefore, I would conclude that the addition of ezetimibe to high intensity statin therapy would likely provide modest benefit while increasing medication expenditure. In accordance with the most recent hyperlipidemia guidelines, if it was felt that high-intensity statin therapy did not reduce LDL cholesterol sufficiently then I think it may be reasonable to consider the addition of non-statin therapy as long as this does not result in the reduction of statin intensity secondary to new adverse events. (7) Ezetimibe Added to Statin Therapy After Acute Coronary Syndrome NEJM 2015; 372(25):2387-2397 Mentor: Dave L. Dixon, PharmD, AACC, FNLA, CDE, CLS, BCPS-AQ Cardiology Presented: September 25, 2015 6) Would you recommend re-instating the LDL thresholds postthis trial? The reduction in LDL cholesterol and improvement in cardiovascular outcomes observed in IMPROVE-IT lend support to the “LDL hypothesis” that more benefit is gained with every decrease in LDL cholesterol. (1) If this is true, then it would make sense to have targets for LDL reduction included in clinical guidelines. However, there is no evidence to date to suggest LDL cholesterol can be lowered pharmacologically to a point that results in adverse effects. Therefore, I would recommend minimum LDL targets to be achieved without a limit on the degree of LDL lowering. References 1) Cannon CP, Blazing MA, Giugliano RP et al. Ezetimibe added to statin therapy after acute coronary syndrome. NEJM 2015; 372(25):2387-2397. 2) Sabatine MS, Giugliano RP, Wiviott SD et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. NEJM 2015;372(16):15001509. 3) Anticipated availability of first-time generics. Pharmacist’s Letter 2015;Detait-Document #310944. Accessed October 10, 2015. 4) Cannon CP, Braunwald E, McCabe CH et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. NEJM 2004;350(15):1495-1504. 5) Micromedex. RED BOOK Online. Ann Arbor (MI): Truven Health Analytics; 2013 [cited 10 Oct 2015]. Available from: www.micromedexsolutions.com. 6) Cannon CP, Blazing MA, Giugliano RP et al. OnTreatment Analysis of the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT). Available from: http://my.americanheart.org/idc/groups/ahamahpublic/@wcm/@sop/@scon/documents/downloadable /ucm_469611.pdf. 7) Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation 2013;129:S1-S45. Kyle D. Thorner, PharmD WakeMed Health & Hospitals