New Approaches to LDL Reduction
Cholesterol Absorption Inhibitors
Inhibition of Cholesterol Absorption and
Production With Ezetimibe/Simvastatin
Simvastatin
Liver
synthesis
Ezetimibe
1000 mg/day
Dietary
cholesterol
~300 mg/day–700 mg/day
Biliary cholesterol
~1000 mg/day
Extrahepatic
tissues
Intestine
Absorption
Excretion
2
Cholesterol Balance in Mice
(µmol/day.100 g body wt)
Liver
VLDL
LDL
Peripheral cells
Forward pathway
LDL
Reverse pathway
Bile
HDL
4
2
5
5
Feces
Diet
10
7
Duodenum
Jejunum
Ileum
3
TICE (µmol/100gr/day)
Ezetimibe strongly increases TICE
bile
Absorption (%)
60
50
40
30
80
60
40
20
0
Control
20
Ezetimibe
10
0
Control
Ezetimibe
TICE
(re)absorption
Feces
8
Control
6
+ Ezetimibe
4
2
0
Control
Ezetimibe
Neutral sterols (µmol/100gr/day)
Chol intake(µmol/100gr/day)
Diet
80
60
40
20
0
Control
Ezetimibe
4
Cholesterol Fluxes in Humans
(mg/day.70 kg body wt)
Liver
VLDL
LDL
Peripheral cells
Forward pathway
LDL
Reverse pathway
Bile
700
1000
HDL
300
700
Feces
Diet
1000
400
Duodenum
Jejunum
Ileum
5
Next Steps
• Assessment of direct intestinal cholesterol excretion in vivo
in humans.
• Determine the contribution of TICE in low and high
absorbers
• Test the effect of pharmacological manipulation in
humans.
6
Cholesterol Absorption Inhibitors Lower
LDL-C and that is Enough in Itself
7
ENHANCE
8
ENHANCE
- Logical Next Step After ASAP Timeline
1995
LIPID (pediatric)
Pravastatin 20-40 mg
Versus
Placebo
2000
ASAP
Atorvastatin 80 mg
Versus
Simvastatin 40 mg
2005
2010
ENHANCE
Simvastatin 80 mg
+ Ezetimibe 10 mg
Versus
Simvastatin 80 mg
Wiegman et al, Efficacy and Safety of Statin Therapy in Children With FH. JAMA 2004; 292(3):331-7
Smilde et al, Atorvastatin versus Simvastatin on Atherosclerotic Progression study. Lancet 2001;357:577-81
9
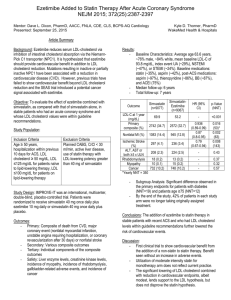
ENHANCE Study Population
Major inclusion criteria
HeFH:
• Genotyping
• Diagnostic criteria WHO
Major exclusion criteria
High-grade carotid stenosis
History carotid endarterectomy
Age 30-75 years
Untreated LDL-C levels > 210 mg/dL
(5.43 mmol/l)
Carotid stenting
Patients on lipid-lowering treatment
LDL-c after wash –out > 210 mg/dL
(5.43 mmol/l)
Congestive heart failure III/IV
Kastelein et al, ENHANCE NEJM 2008;358 ;1431-43
NO MINIMAL CAROTID IMT
ENTRY CRITERIA
10
ENHANCE
Study Design
Pre-randomization Phase
FH:
LDL-c ≥ 210 mg/dL
Screening and
Fibrate
Washout
Placebo LeadIn/ Drug
Washout
R
A
N
D
O
M
I
Z
A
T
I
O
N
Ezetimibe 10 mg-Simvastatin 80 mg
Simvastatin 80 mg
IMT assessment
-10 to -7
Weeks
-6
0
3
6
9
12
Months
15
18
21
24
11
Baseline Characteristics
Simvastatin Monotherapy Simvastatin plus
Ezetimibe
All randomized patients
n=363
n=357
P-value
Age (yr)
45.710.0
46.19.0
0.69
Male sex no. (%)
179(49%)
191 (54%)
0.26
Body-mass index
26.74.4
27.44.6
0.047
5(1%)
8 (2%)
0.38
Hypertension
51 (14%)
67 (19%)
0.09
Current smoking
104 (29%)
102 (29%)
0.98
26 (7%)
14 (4%)
0.06
297 (82%)
286 (80%)
0.56
Systolic mm Hg
12415
12515
0.31
Diastolic mm Hg
7810
789
0.41
History of diabetes
History of MI
Prior use of statins
Kastelein et al, ENHANCE NEJM 2008;358 12
;1431-43
LDL-Cholesterol
10
Percentage change from baseline
0
10
20
30
40
50
60
70 0
24 months
(mg/dL)
193 ± 60
Decrease
(%)
Simva
Baseline
(mg/dL)
318 ± 66
Eze-Simva
319 ± 65
141 ± 53
-56%
-40%
P<0.01
-16.5 % incremental
reduction
6
12
Months
18
24
Simva
Eze-Simva
Kastelein et al, ENHANCE NEJM 2008;358 13
;1431-43
ENHANCE
hsCRP
10
Median percent change from Baseline
0
p < 0.01
-10
Simva
Baseline
(mg/L)
1.7 (0.8-4.1)
24 months
(mg/L)
1.7(0.8-3.9)
Eze-Simva
1.2(0.6-2.4)
0.9(0.5-1.9)
-20
-30
-26 % incremental
reduction
-40
-50
-60
-70
-80
3
6
12
Months
18
24
Simva
Eze-Simva
14
Mean cIMT During 24 Months of Therapy
Longitudinal, Repeated Measures Analysis
0.80
Mean IMT (mm)
0.75
P=0.88
0.70
0.65
0.60
6
12
Months
18
24
Simva
Eze-Simva
15
Kastelein et al, ENHANCE NEJM 2008;358 ;1431-43
Possible Explanations for the Absence of an
Incremental Reduction in cIMT
Measurement Technique
Technique not accurate enough to reflect changes
in atherosclerotic burden?
The Compound
Ezetimibe lacks vascular benefit despite the
observed LDL-c and hsCRP reduction
The Population
At too low a risk to detect changes, which would
limit the ability to detect a differential response
16
The Trial Design and Population
To have any chance of success using cIMT to demonstrate that one treatment is
better than another one of two critical factors must be present – preferably both:
• The ‘control’ group must show significant progression – if not then only
significant regression in the ‘test’ group can result in a positive trial
• The population studied must have significant and quantifiable lipid rich
intima – if minimal or no significant atherosclerosis is present then only
possible change to be assessed is progression
17
Critical Factors for Successful cIMT Trial
ASAP - 1997
0.95
progression
P <0.05
0.90
Simva LDLc -40%
Atorva LDLc -52%
0.85
cIMT mm
ASAP
0.80
0.75
0.70
Simva/Control progressed;
atorva/Test stable/regressed
SUCCESS!!
0.65
regression
0
1
2
years
18
Critical Factors for Successful cIMT Trial
ASAP - 1997
0.95
ASAP
progression
0.90
Simva LDLc -40%
Atorva LDLc -52%
cIMT mm
0.85
0.80
0.75
ENHANCE - 2003
P= ns
0.70
ENHANCE
Simva LDLc -40%
Simva/Eze LDLc -57%
0.65
regression
0
1
2
years
19
ASAP and ENHANCE
Baseline cIMT in LIPID (Pediatric)
ASAP
ENHANCE
LIPID (pediatric)
Frequency
Baseline
mean cIMT
LIPID (pediatric)
0.4
0.8
1.2
1.6
Mean CIMT (mm)
2.0
0.495±0.050
ASAP
0.92±0.20
ENHANCE
0.70±0.13
2.4
20
What About the Trial Indicating Potential Harm
from Increased CVD Events?
• Although
ENHANCE was a relatively small trial in low risk
FH patients was there any evidence from the CVD events
that addition of ezetimibe caused harm?
• Can one even pick up such a signal from such small trials
as ENHANCE in this FH population?
21
CVD Events – Recent FH cIMT Trials:
RADIANCE I (CETPi) and CAPTIVATE
(ACATi)
RADIANCE I
Incidence of CVD events (%)
Atorva
Atorva + Torcet
Statin
Statin+ ACATi
(n=454)
(n=450)
(n=438)
(n=443)
CVD death/MI/
Revasc/Stroke
CAPTIVATE
`p<0.02
`p<0.05
11 (2.4%)
23 (5.1%)`
15 (3.4%)
28 (6.3%)`
Thus even small studies (700-900 patients) in FH appear to be able to
detect potential CVD harm
*Kastelein et al NEJM 2007; 356:1620-30
**Meuwese MC et al JAMA in press
22
Conclusion from ENHANCE
While the results of ENHANCE have been less than
optimal for the sponsors of the trial they actually carry very
good news for those with FH, and all patients on long term
lipid lowering therapy, in that the data would seem to
strongly indicate that even moderate, long term LDLc
lowering dramatically reduces the atherosclerotic burden,
at least in carotid arteries, and virtually halts progression of
the underlying disease.
23