HS Calorimeter Engineering Design Handout v1.1
advertisement

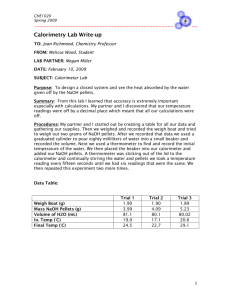
For Students Engineering Design in Oregon Science Classrooms Name __________________________________________________________________ Page 1 of 4 Period _________ Engineering Design Handout for Calorimeters DESIGN YOUR OWN CALORIMETER 1. Identify your problem with a problem statement. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ 2. Identify your criteria for solving this problem. List them in order of priority. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ 3. Identify any constraints (materials, size, etc.) __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ 4. Are there any trade-offs to keep in mind? __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ For Students Engineering Design in Oregon Science Classrooms Page 2 of 4 5. Using your knowledge from the “Wait, What Just Happened?” activity, and the above Engineering Design strategies, design your own calorimeter in the box below. Propose this solution to your teacher. For Students Engineering Design in Oregon Science Classrooms Page 3 of 4 USING YOUR CALORIMETER TO MEASURE HEAT TRANSFER Now that you have gotten your proposal approved, and have built your calorimeter, you are ready to test your design, and see how much heat you will lose. 1. Measure 100ml of room temperature water into a beaker. Record the volume in the table below. Fill in the mass by assuming one ml of water has 1 gm of mass. 2. Pour the room-temperature water into your calorimeter and wait a few minutes to allow the water and the calorimeter to reach the same temperature. Record the temperature in the table below. 3. Measure 100ml of hot water in a beaker. 4. Quickly pour the hot water into the calorimeter and close the lid. Watch the thermometer as the temperature rises. Stir the water using the thermometer to uniformly mix it. Wait until the temperature stabilizes at a single temperature on the thermometer. Record this final temperature value in the table below. 5. Now calculate the energy transfer below. Volume Mass Initial Temperature Final Temperature Room Temp. Water Calculations 𝑄𝑒𝑥𝑝 = 𝑛𝐶𝑃 ∆𝑇 𝑚𝑎𝑠𝑠 𝑛 = 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 The molar mass of water is 18.0 grams per mole. The heat capacity of water is 𝐶𝑃 = 4.186 J/(g·C) For Students Engineering Design in Oregon Science Classrooms Page 4 of 4 MEASURING ENERGY TRANSFER Use you calorimeter to measure how much energy is consumed by the dissolution of KCl (potassium chloride) salt in water. 1. Fill your calorimeter with approximately 100ml of water. Let it sit for a few minutes to allow the water and the calorimeter to reach the same temperature. 2. Record the water temperature below. This will also be your initial temperature for your device. 3. Weigh out approximately 25g of KCl crystals. Record the mass below. 4. Pour your KCl into your calorimeter and seal the top. Make sure all the salt has dissolved by stirring with the thermometer and listening for it “scratching” at the bottom of the cup while stirring. Record the final temperature at which it stops getting colder and you are fairly certain all the salt has dissolved. 5. Use the formulas to calculate the measured heat transfer Qexp and the predicted heat absorbed, Qideal. in kJ. (Use additional sheets of paper if need more room.) Volume H2O: Mass H2O: Mass KCl: Initial Temperature: Final Temperature: Calculations 𝑄𝑒𝑥𝑝 = 𝑚𝐶𝑃 ∆𝑇 The heat capacity of water is 𝐶𝑃 = 4.186 J/(g·C) 𝑄𝑖𝑑𝑒𝑎𝑙 = −∆𝐻𝑛 𝑛 = 𝑚𝑎𝑠𝑠 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 The molar mass for KCl is 74.6 g/mole. ΔH for KCl is 17.2 kJ/mol.