Experiment 7: Calorimetry – Lab Notebook Prep
advertisement

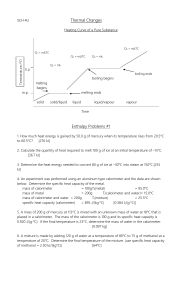
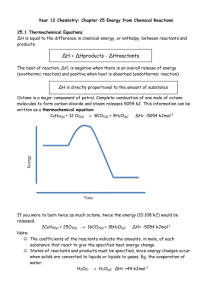
Experiment 7: Calorimetry – Lab Notebook Prep Note: Prepare your lab notebook with these tables, but you will not be submitting these pages from your lab manual. Instead, you will submit a formal lab report which includes the data and results in these tables. Mass of empty Calorimeter #1 + cover: ________________________________ Experimental Determination of the Hneut for HCl(aq)-NaOH(aq) and for HNO3(aq)-NaOH(aq) sodium hydroxide hydrochloric acid nitric acid 50.00 mL 50.00 mL 50.00 mL Molarity of the solution Volume of solution used in each trial HCl(aq)-NaOH(aq) Trial #1 Trial #2 Initial Temperature of HCl(aq) Initial Temperature of NaOH(aq) Average Initial Temperature Mass of Calorimeter #1 + solution + cover (after reaction) Mass of empty Calorimeter #1 + cover (from part A) Mass of solution* * Use Mass of empty Calorimeter #1+ cover from Part A to calculate HNO3(aq)-NaOH(aq) Trial #1 Trial #2 Initial Temperature of HNO3(aq) Initial Temperature of NaOH(aq) Average Initial Temperature Mass of Calorimeter #1 + solution (after reaction) Mass of solution* *Use Mass of empty Calorimeter #1+ cover from Part A to calculate For each trial, attach the Data Table obtained using the Vernier LabQuest system, along with the titled graph with labeled axes that shows the regression line and the y-intercept with the maximum temperature (Tfinal). Show calculations for each step of the HCl(aq)-NaOH(aq) and the HNO3(aq)-NaOH(aq) neutralization reactions in your lab notebook. Results Table to Determine Heat of Neutralization for the HCl(aq)-NaOH(aq) Reaction Trial #1 Trial #2 Maximum Temperature (y-intercept) from the plot Change in Temperature (T) qsolution (in kJ) qreaction (in kJ) # of moles of H2O produced Hneut (in kJ/mol H2O) Average Hneut (in kJ/mol H2O) Results Table to Determine Heat of Neutralization for the HNO3(aq)-NaOH(aq) Reaction Trial #1 Maximum Temperature (y-intercept) from the plot Change in Temperature (T) qsolution (in kJ) qreaction (in kJ) # of moles of H2O produced Hneut (in kJ/mol H2O) Average Hneut (in kJ/mol H2O) Trial #2