1b - bomb calorimeter
advertisement
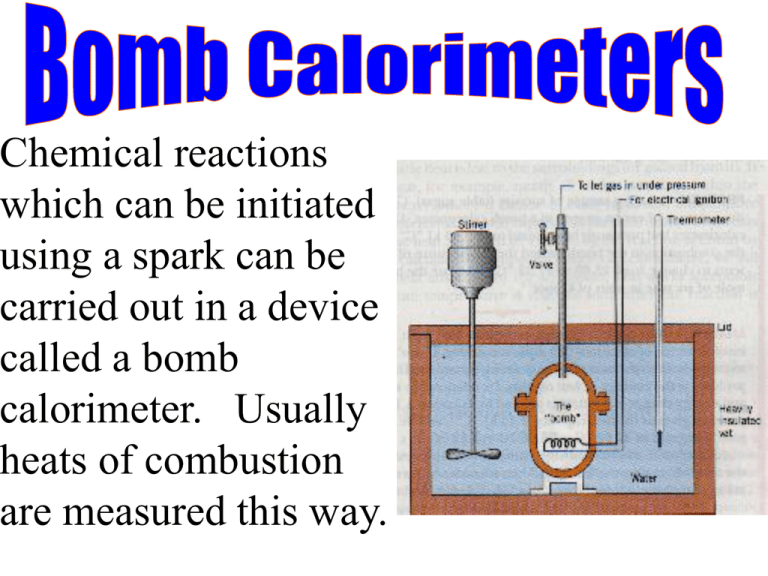
Chemical reactions which can be initiated using a spark can be carried out in a device called a bomb calorimeter. Usually heats of combustion are measured this way. The substance to be burned is massed into the “bomb, which is fitted with a device that can deliver a spark and with a tube that can deliver oxygen under pressure. The bomb is then sealed and immersed in a well-insulated vat of water. Oxygen is let into the bomb, the spark generated, the reaction occurs, and no products escape as the heat is generated. The heat warms the bomb and thus the water surrounding it. The “stuff” absorbing the heat is not only the water, but also anything immersed in it. It includes the thermometer and the stirrer which ensures that any heat is uniformly distributed before the final temperature is read. Before a bomb calorimeter is used its specific heat capacity must be determined. Sample Problem A 1.32 g sample of sucrose, C12H22O11 is burned in the presence of excess oxygen in a bomb calorimeter. The heat capacity of the calorimeter is 9.43 kJ/oC. The heat liberated by the combustion in the bomb caused the temperature of the calorimeter’s contents to change from 25.00 oC to 27.32 oC. Calculate the heat of combustion per mole of sucrose in units of kJ/mol.