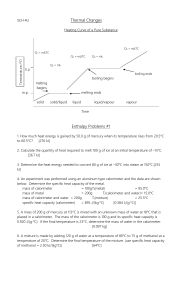
Thermodynamics PowerPoint – Notes Energy is... The ability to do
advertisement

Thermodynamics PowerPoint – Notes Energy is... • The ability to do ____________________. • ____________________. • Made of ____________________and ____________________. • A ____________________function: values that depend on the state of the ____________________, and not on how that state was reached. • Independent of the ____________________, or how you get from point A to B. • Work is a ____________________acting over a ____________________. • Heat is ____________________transferred between objects because of ____________________difference. The Universe • Is divided into ________________________________________. • The ____________________and the ____________________. • The ____________________is the part you are concerned with. • The ____________________are the rest. • ____________________processes release energy to the surroundings. • ____________________processes absorb energy from the surroundings. Units of Heat • ____________________ (cal) The quantity of heat required to change the temperature of one • ____________________of water by one degree ____________________. • ____________________ (J) ____________________ unit for heat • Direction Every energy measurement has three parts. 1. A unit (____________________or ____________________). 2. A ____________________how many (magnitude). 3. A ____________________to tell direction. • negative - ____________________ • positive- ____________________ First Law of Thermodynamics • The energy of the universe is ____________________. • Law of ____________________of energy. • q = ____________________ • Take the ____________________ point of view to decide signs. • q is negative when the system ____________________heat energy • q is positive when the system ____________________heat energy Heat Capacity • The quantity of heat required to change the temperature of a system by one degree. • Three “types” with different systems: ________________________________________Capacity ____________________Heat Capacity (we won’t cover this one in Physics) ________________________________________ Heat Capacity Types Specific heat capacity • System is one ____________________of substance • Units are ____________________ • q = ________________________________________ • m = ____________________ • C = ________________________________________ • ΔT = ____________________ temperature – ____________________ temperature • q = ________________________________________ Heat Capacity Types Heat capacity of an object • Specific for a given ____________________or ____________________ So it is not mass dependent • Units are ____________________ • q = ____________________ • C = ________________________________________ • DT = ____________________temperature – ____________________temperature • q = ____________________ Heat Capacity Types • So how do you know which one to use when? • Look at the ________________________________________ • Select the value with appropriate ____________________to cancel Example • The specific heat of graphite is 0.71 J/gºC. Calculate the energy needed to raise the temperature of 75 kg of graphite from 294 K to 348 K. • q = ____________________________________________________________ • = ____________________ Note that ΔT oC and ΔT K are ____________________, since they are the same “____________________”. Example When a piece of copper (5.0 g) is heated for 2.0 seconds, and 100 J of heat energy is transferred to the copper, the temperature increases from 20.0 ˚C to 71.9 ˚C. What is the specific heat of the copper? m = 5.0 g; ΔT = (71.9 – 20.0) = 51.9˚C; q = 100 J; t = 2.0 s q = mCΔT; ____________________________________________________________ ____________________is irrelevant. Example If 10.0 g of Cu is heated for 2.0 seconds from 20.0 ˚C and 200 J of heat are absorbed, what is the final temperature of the block? (Let’s assume time is again ____________________.) m = ____________________; Ti = ____________________; Tf = ?; CCu = ____________________; q = ____________________ q = ____________________________________________________________ Tf = ________________________________________ Calorimetry • Measuring ____________________changes to calculate ____________________changes. • Use a ____________________. • Two kinds – Constant ____________________calorimeter (called a ____________________ calorimeter) – Constant ____________________calorimeter (called a ____________________calorimeter) Calorimetry • A coffee cup calorimeter measures ____________________and calculates ____________________. • An ____________________ cup, full of ____________________ (constant pressure, variable volume) • ____________________is the surroundings in which the system changes • Calculate the heat ____________________of water. • The specific heat of water is ____________________ • Heat of water qH2O ____________________ • ____________________ + ____________________ = 0 • ____________________ + ____________________ = 0 • ____________________ = ‒ ____________________ In interactions between a ____________________and its ____________________the total energy remains ____________________— energy is neither ____________________nor ____________________. Law of ____________________of ____________________ Coffee Cup Calorimeter • A simple calorimeter. – Well ____________________and therefore ____________________. – Measure ________________________________________. • ____________________ = ____________________ Determination of Specific Heat (note temperature and mass values) Determining Specific Heat from Experimental Data Use the data presented on the last slide to calculate the specific heat of lead. ____________________ = ____________________ q H2O = ____________________________________________________________ q H2O = ____________________ q Pb = ____________________ c Pb =____________________ Equal masses of liquid A, initially at 100 ˚C, and liquid B, initially at 50 ˚C, are combined in an insulated container. The final temperature of the mixture is 80 ˚C. Which has the larger specific heat capacity, A or B? mA = ____________________________________________________________ qA = ____________________________________________________________ CA/CB = ____________________________________________________________ You do one… When 86.7 grams of water at a temperature of 73.0 ˚C is mixed with an unknown mass of water at a temperature of 22.3 ˚C the final temperature of the resulting mixture is 61.7 ˚C. What was the mass of the second sample of water? States of Matter General Heating Curve: Draw and label the diagram. Changes of State for Water Heat of ____________________ (liquid <----> gas) H2O(l) ↔ H2O(g) Hv = ____________________ J/g Heat of ____________________ (solid <----> liquid ) H2O(s) ↔ H2O(l) Hf = ____________________ J/g Heating Curve for Water q = mCT Total heat ____________________ by the water as it is warmed can be determined by finding the heat involved in each “____________________” of the process. ____________________ = qtotal Note: ____________________ = ____________________ = ____________________ q = ____________________ ____________________ = Hphase change Example: Calculate q for the process in which 50.0 g of water is converted from liquid at 10.0°C to vapor at 125.0°C. Example: What is the heat of fusion of lead in J/g if 6.30 kilojoules of heat are required to convert 255 grams of solid lead at its melting point into a liquid? Example: What quantity of heat is required to heat 1.00 g of lead from 25 ˚C to the melting point (327 ˚C) and melt all of it? (The specific heat capacity of lead is 0.159 J/g • K and it requires 24.7 J/g to convert lead from the solid to the liquid state.)