Molecular Geometry Guide
advertisement
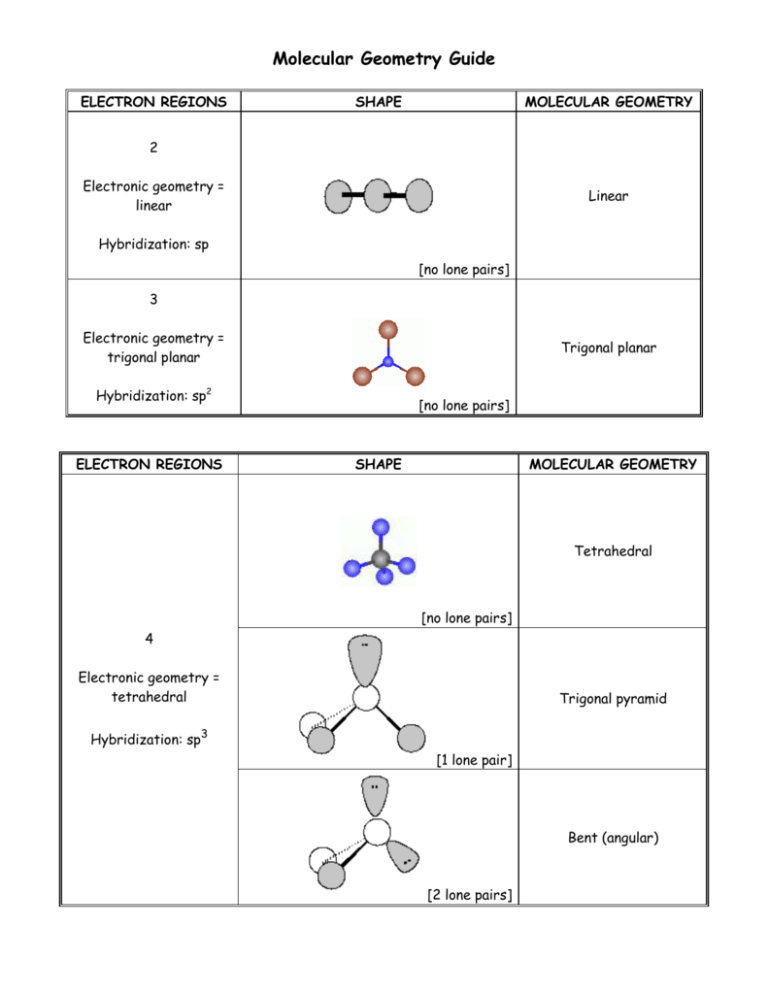
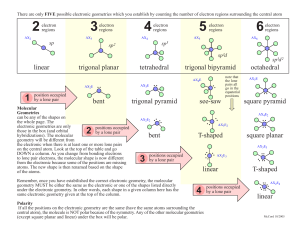
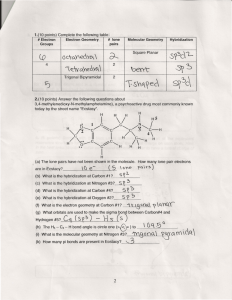
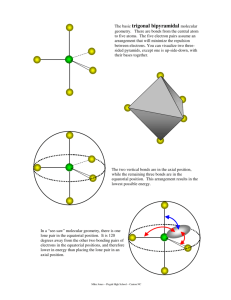
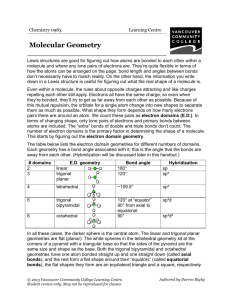
Molecular Geometry Guide ELECTRON REGIONS SHAPE MOLECULAR GEOMETRY 2 Electronic geometry = linear Linear Hybridization: sp [no lone pairs] 3 Electronic geometry = trigonal planar Trigonal planar Hybridization: sp2 ELECTRON REGIONS [no lone pairs] SHAPE MOLECULAR GEOMETRY Tetrahedral [no lone pairs] 4 Electronic geometry = tetrahedral Trigonal pyramid Hybridization: sp3 [1 lone pair] Bent (angular) [2 lone pairs] ELECTRON REGIONS SHAPE MOLECULAR GEOMETRY Trigonal bipyramid [no lone pairs] See-saw 5 Electronic geometry = trigonal bipyramid [1 lone pair] Hybridization: sp3d T-shape [2 lone pairs] Linear [3 lone pairs] ELECTRON REGIONS SHAPE MOLECULAR GEOMETRY Octahedral [no lone pairs] 6 Electronic geometry = octahedral Hybridization: sp3d2 Square pyramidal [1 lone pair] Square planar [2 lone pairs] Polarity is determined by the electron regions around the central atom. If the regions are identical and everything is symmetrical then the molecule is most likely non-polar. However, if the regions are NOT identical or the molecule is NOT symmetrical the molecule is most likely polar. The importance of polarity determines the phase change temperatures of molecules (melting point, boiling point, etc.) and solubility (whether or not the molecule is soluble or insoluble in certain substances). The rule of thumb for solubility is “like dissolves like” – nonpolar substances dissolve in nonpolar solvents while polar substances dissolve in polar substances. Water will dissolve most ionic salts and polar molecules.