si worksheet 18
advertisement
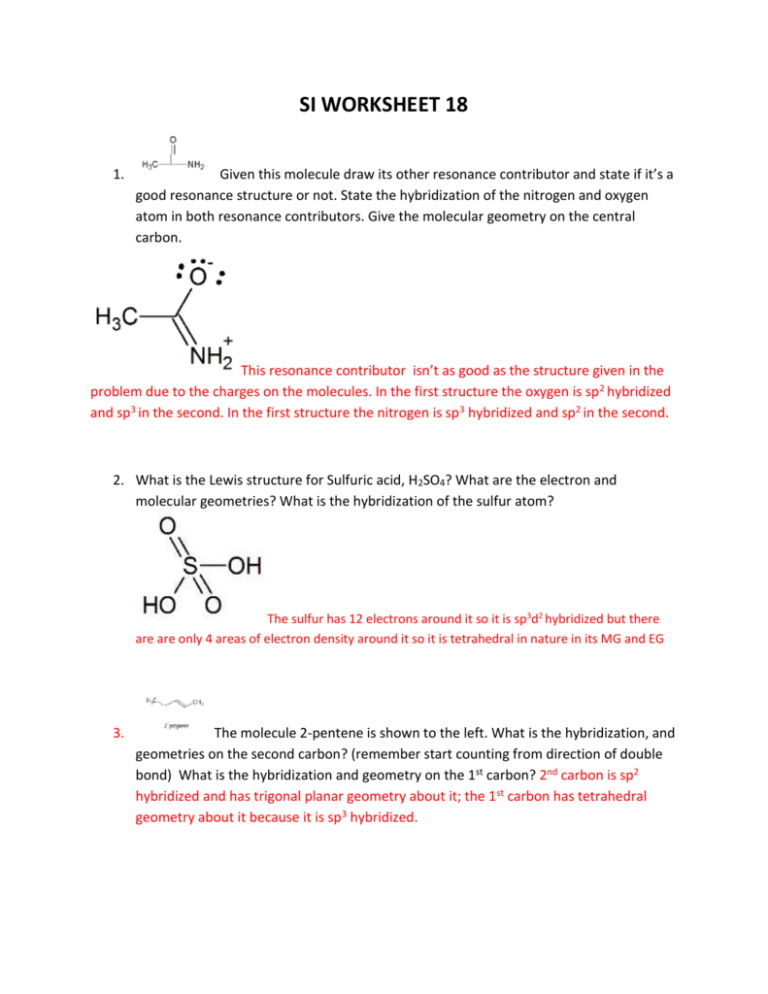
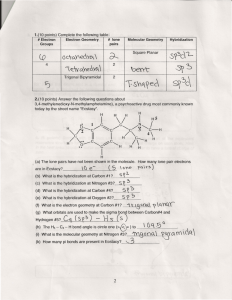
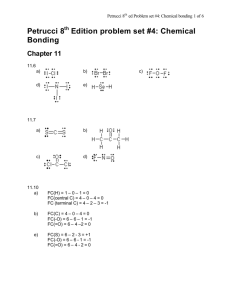
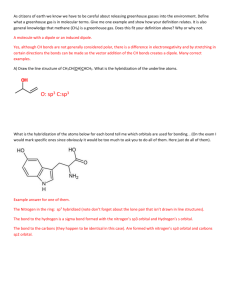
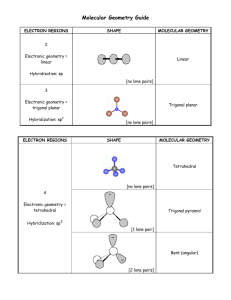
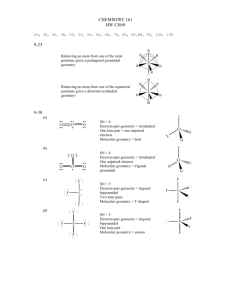
SI WORKSHEET 18 1. Given this molecule draw its other resonance contributor and state if it’s a good resonance structure or not. State the hybridization of the nitrogen and oxygen atom in both resonance contributors. Give the molecular geometry on the central carbon. This resonance contributor isn’t as good as the structure given in the problem due to the charges on the molecules. In the first structure the oxygen is sp 2 hybridized and sp3 in the second. In the first structure the nitrogen is sp 3 hybridized and sp2 in the second. 2. What is the Lewis structure for Sulfuric acid, H2SO4? What are the electron and molecular geometries? What is the hybridization of the sulfur atom? The sulfur has 12 electrons around it so it is sp3d2 hybridized but there are are only 4 areas of electron density around it so it is tetrahedral in nature in its MG and EG 3. The molecule 2-pentene is shown to the left. What is the hybridization, and geometries on the second carbon? (remember start counting from direction of double bond) What is the hybridization and geometry on the 1st carbon? 2nd carbon is sp2 hybridized and has trigonal planar geometry about it; the 1 st carbon has tetrahedral geometry about it because it is sp3 hybridized. 4. XeF4 has what molecular and electron geometry? What about BrCl5 ? XeF4 has a molecular geometry of square planar and an electron geometry of octahedral; BrCl5 has a molecular geometry of triangular bipyramidal and an EG of octahedral. 5. Compare H2S to H2O: Which one has the larger bond angle and how do you know? *molecules not shown* but you guys know how to draw them. Water would have the larger bond angle because the central atom of hydrogen sulfide (Sulfur) is larger than the central atom of water (oxygen) and so it results in a smaller bond angle. 6. Acetylene (Ethylene) is C2H2 how many sigma and pi bonds are in this molecule? What is the hybridization of the carbon atoms? Both Carbons are sp hybridized. There are 3 sigma bonds and 2 pi bonds in this molecule 7. Acetylcholine is shown to the left. What is the hybridization of the carbonyl carbon and the nitrogen? How many pi bonds? Sigma? The carbonyl carbon is sp2 hybridized and the nitrogen is sp3 hybridized because it has 4 areas of electron density. In this molecule there are 21 sigma bonds and 1 pi bond.