Lesson 8.6 VSEPR
advertisement

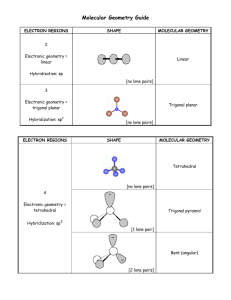
Lesson 8.6 The Valence-Shell Electron-Pair Repulsion (VSEPR) Model Suggested Reading Zumdahl Chapter 8 Section 8.13 Essential Question What are the three-dimensional structures of molecules? Learning Objective Predict the shape and bond angles of molecules using the valence shell electron pair repulsion (VSEPR) model. Introduction Molecular geometry is the general shape of a molecule, as determined by the relative positions of the atomic nuclei. The valence shell electron pair repulsion (VSEPR) model allows you to predict the molecular geometry of a molecule or ion from Lewis structures. Keep in mind that VSEPR does not explain chemical bonding, but it does help us to understand molecules after bonds have formed. The VSEPR Model The valence shell electron pair repulsion (VSEPR) model predicts the shapes of molecules by arranging valence-shell electron pairs (both bonding and non-bonding) so that the electron pairs keep as far away from one another as possible thereby minimizing electron-pair repulsions. Watch this video about VSEPR to review the five basic electronic geometries (Great video, don't skip!): YouTube Video https://www.youtube.com/watch?v=keHS-CASZfc The basic electronic and molecular geometries for molecules with 2, 3, 4, 5, and 6 regions of electron density around a central atom are shown below. Please note that the number of regions of electron density are sometimes referred to as steric number, as shown below. Molecular versus Electronic Geometry In the chart above there is more than one geometry for each steric number greater than two. The different geometries are determined by the number of lone pairs around the central atom. The electronic geometry is the shape the molecule takes when both the bonding and nonbonding electrons are considered. The molecular geometry is is the electronic geometry minus the lone pairs, because the lone pairs are "invisible". The molecular geometry is what is actually observed. For example, consider ammonia (NH3) Ammonia has a steric number of 4, so there are four regions of electron density around the central atom; three bonding pairs and one lone pair. The electronic geometry is tetrahedral. However, if we subtract the lone pair, the molecular geometry is trigonal pyramidal. When predicting the molecular geometry, be careful not to ignore the lone pair. In this example, ignoring the lone pair would result in a trigonal planar molecular geometry, which is not correct! Notice that the bond angles of the molecular geometry are slightly less than that of the tetrahedral molecular geometry. This reflects that fact that lone pairs are more repulsive than bonded pairs. The following chart may help you to better visualize the differences between molecular and electronic geometry for each steric number. Predicting Molecular Geometry Using the VSEPR Model 1. 2. 3. 4. Write the Lewis structure. Determine how many electron pairs are around the central atom. Count a multiple bond as one one pair. If resonance occurs, use one resonance formula to determine this number. Determine the electronic geometry that corresponds to the number of electron pair, and arrange the electron pair accordingly. Obtain the molecular geometry by "subtracting" the lone pairs. Example: Predicting Molecular Geometries Predict the geometry of the following molecules or ions, using VSEPR: a) BeCl2 b) NO2Solution a) BeCl 2 Step 1: Write the Lewis structure (Recall that Be can have less than an octet while Cl always obeys the octet rule). Step 2: Determine the number of electron pairs around the central atom. 2 Step 3: Determine the electron geometry. Linear Step 4: Subtract the lone pairs to determine the molecular geometry. There are no lone pairs, so the molecular geometry is also linear. b) NO 2 Step 1: Write the Lewis structure. Step 2: Using only one resonance structure, determine the number of electron pairs around the central atom counting the multiple bond as one pair. 3 Step 3: Determine the electron geometry. Trigonal planar Step 4: Subtract the lone pairs to determine the molecular geometry. Bent HOMEWORK: Practice exercises 9.1 & 9.2, book questions page 386 questions 91-98 **you do not have to include bond angles in your drawings.