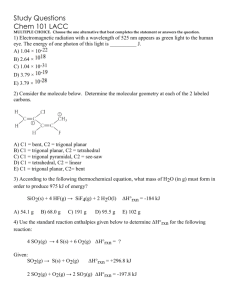
File - Chris Cunnings
advertisement

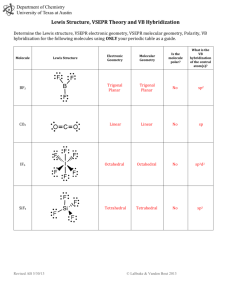
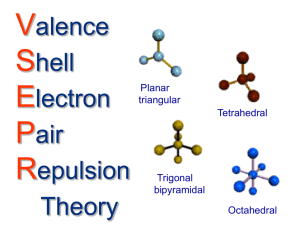
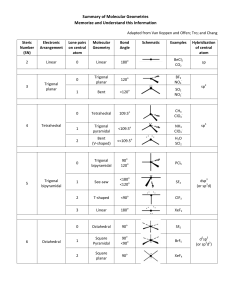
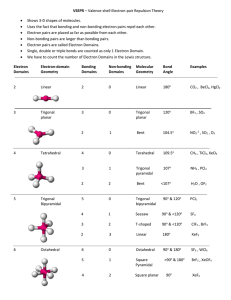
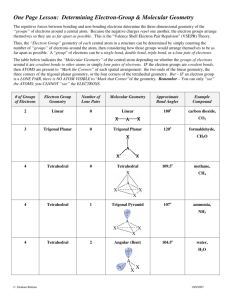
Covalent bonding and VSEPR Steps for determining molecular geometry 1) Draw the Lewis Structure for the molecule (review the correct steps for drawing Lewis Structures if needed) 2) Count the number of “things” that surround the central atom a. “THINGS” include i. Bonds (doesn’t matter if it is a single, double, or triple bond…just count the total bonding domains) ii. Electron pairs 3) Determine what structure the molecule is based on a. If there are just TWO total “things”, it is based on the “LINEAR” electron domain geometry b. If there are THREE total “things”, it is based on the “TRIGONAL PLANAR” electron domain geometry c. If there are FOUR total “things”, it is based on the “TETRAHEDRAL” electron domain geometry d. If there are FIVE total “things”, it is based on the “TRIGONAL BIPYRAMIDAL” electron domain geometry e. If there are SIX total “things”, it is based on the “OCTAHEDRAL” electron domain geometry 4) Which atoms are replaced by lone electron pairs? 5) What is the final structure, and what are the bond angles? a. Final structure possibilities from the possible structures listed in step 3: (bonds/unshared pairs of electrons) i. TWO things: 1. Just linear (2/0) ii. THREE things: 1. Trigonal Planar (3/0) 2. Bent (2/1) iii. FOUR things: 1. Tetrahedral (4/0) 2. Trigonal Pyramidal (3/1) 3. Bent (2/2) iv. FIVE things: 1. Trigonal Bipyramidal (5/0) 2. Seesaw (4/1) 3. T-Shaped (3/2) 4. Linear (2/3) v. SIX things: 1. Octahedral (6/0) 2. Square Pyramidal (5/1) 3. Square planar (4/2) b. Bond angles: i. Linear = 180 degrees ii. Trigonal Planar = 120 degrees iii. Trigonal pyramidal = a little less than 120 degrees…usually about 105 degrees iv. Tetrahedral = 109.5 degrees v. Trigonal bipyramidal = think of it as a LINEAR combined with a TRIGONAL PLANAR vi. Octahedron = 90 degrees Do Molecular Geometry Practice Problems On The Pages That Follow! CO2 BF3 CH4 H2O AsF3 BeH2 SeO2 SCl2 SeF6 IF3 ICl4- SO2 NO3- HCN CI4 PCl5 CO32- BH4- BrF5 KrF4 SF6 TeF4 TeH2 ClF3 XeF2 KrF4 CH2Cl2 BrO4-








![Which is the correct Lewis structure for the nitrate ion, [NO3]– ? a) b](http://s3.studylib.net/store/data/008121614_1-3f41411d21eef682c95d3c7778684719-300x300.png)